Abstract
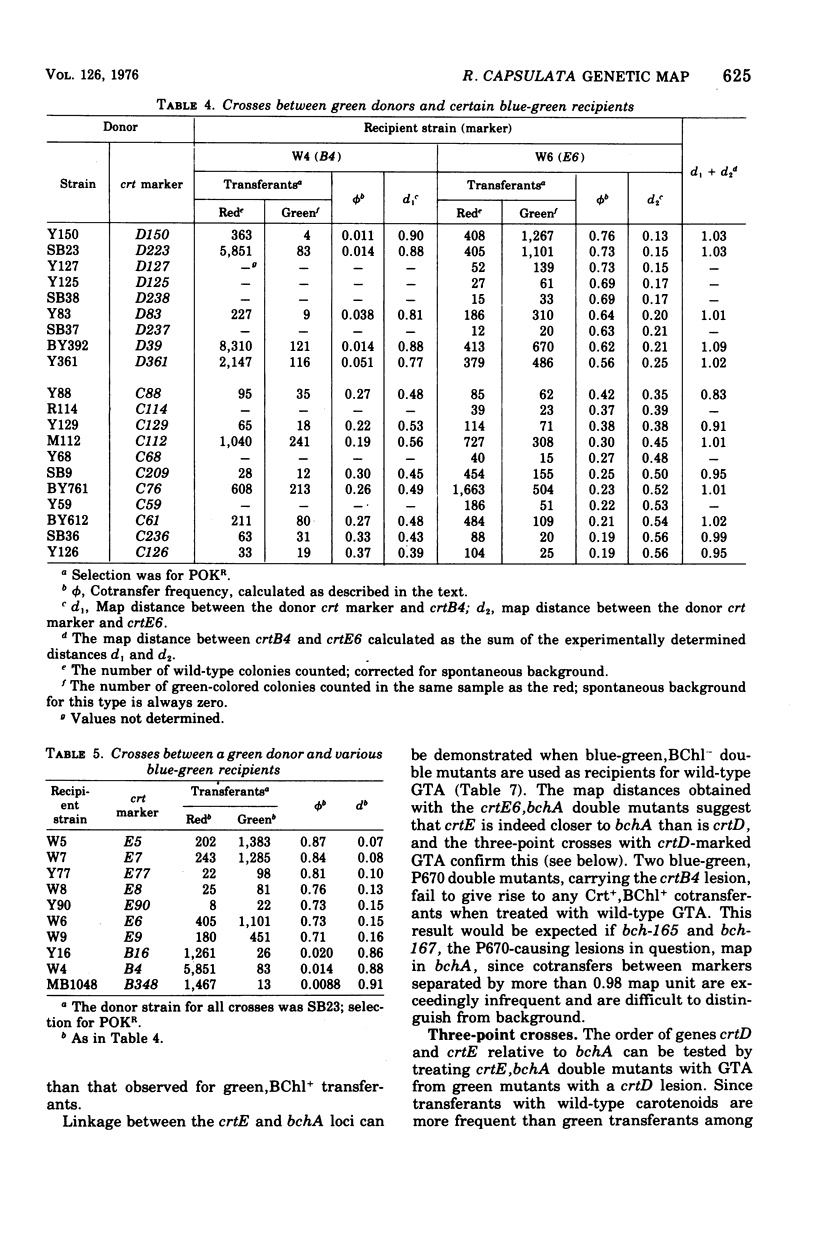
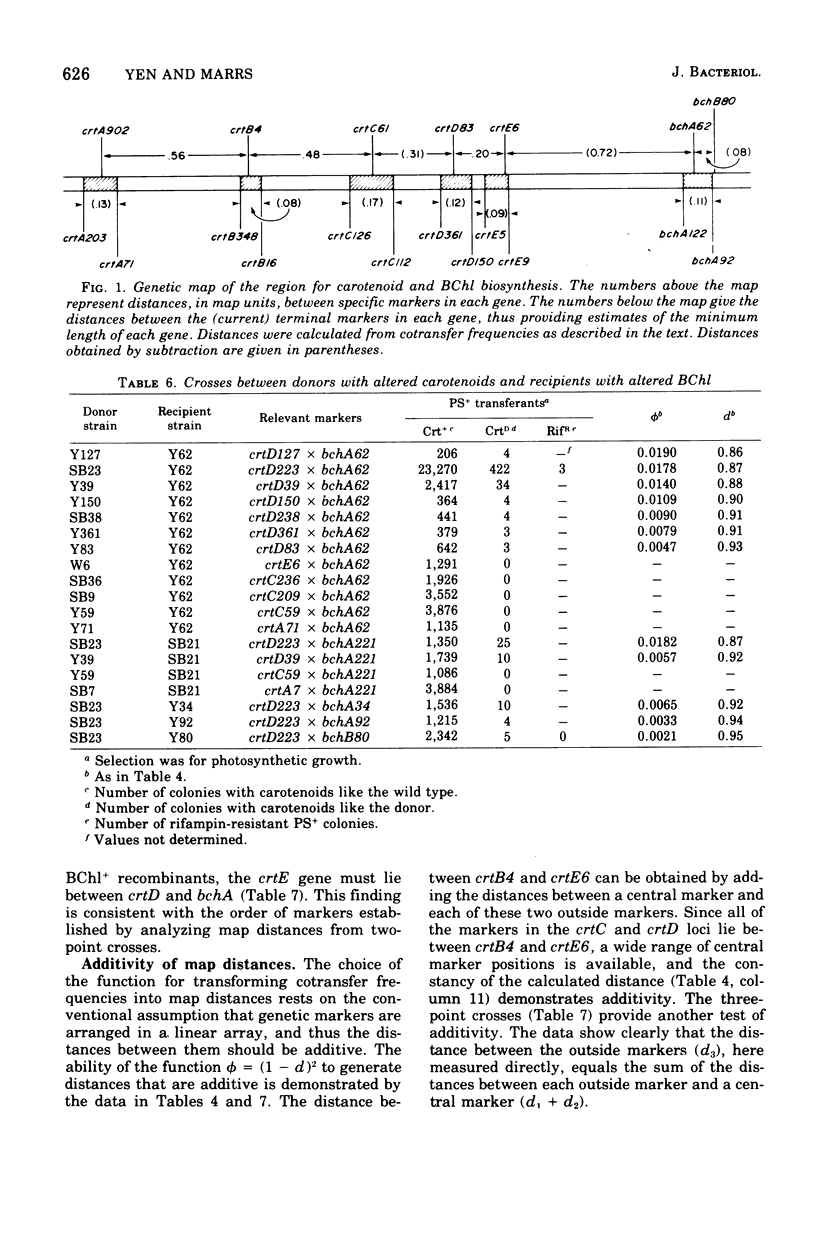
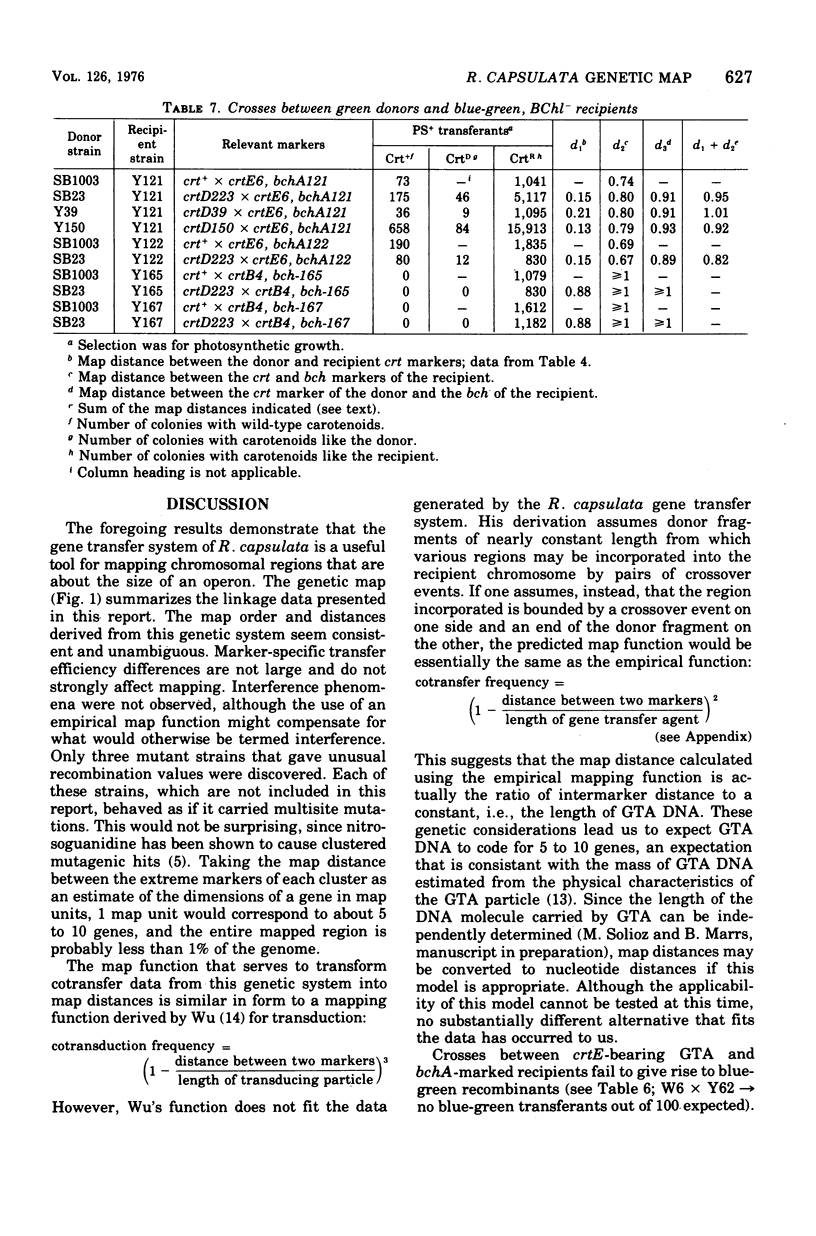
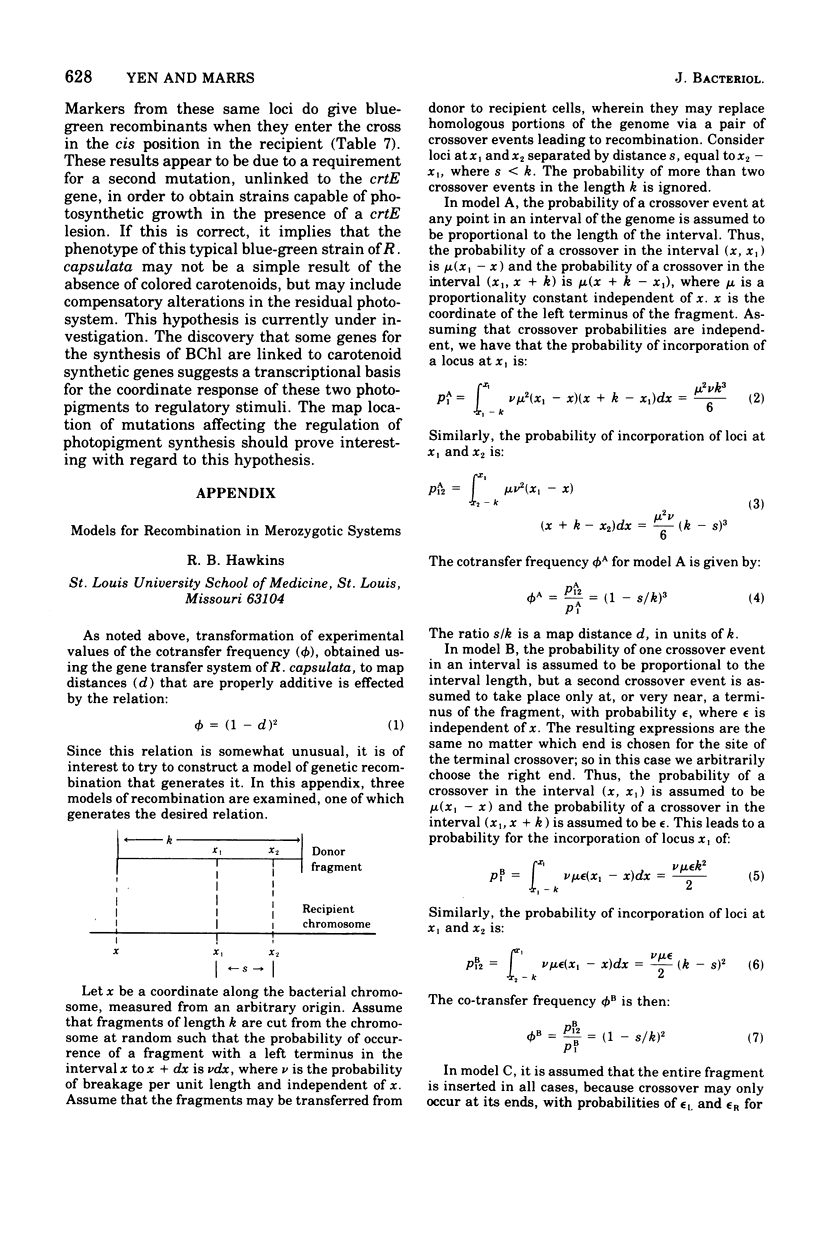
The recently discovered gene transfer system of Rhodopseudomonas capsulata was used to construct a genetic map of a region concerned with bacteriochlorophyll and carotenoid production. Mutants blocked in the biosynthesis of these compounds were isolated, and each was characterized on the basis of pigments accumulated during growth under low pO2. One-point, two-point, three-point, and ratio test crosses were performed between various mutant strains, and the results were amenable to conventional genetic analyses. A mapping function was found that related cotransfer frequency to map distance. Seven clusters of mutations, five affecting carotenoid and two affecting bacteriochlorophyll biosynthesis, were arranged in one linkage group. Each cluster of mutations is thought to represent a gene. The length of the mapped region is estimated to be less than 1% of the genome. Cotransfer is observed between markers separated by about 5 to 10 genes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Kaplan S. Isolation and fractionation of the photosynthetic membranous organelles from Rhodopseudomonas spheroides. J Bacteriol. 1971 Oct;108(1):465–473. doi: 10.1128/jb.108.1.465-473.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITHS M., STANIER R. Y. Some mutational changes in the photosynthetic pigment system of Rhodopseudomonas spheroides. J Gen Microbiol. 1956 Jul;14(3):698–715. doi: 10.1099/00221287-14-3-698. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Lascelles J., Altshuler T. Some properties of mutant strains of Rhodopseudomoas spheroides which do not form bacteriochlorophyll. Arch Mikrobiol. 1967;59(1):204–210. doi: 10.1007/BF00406333. [DOI] [PubMed] [Google Scholar]
- Lascelles J. The regulation of heme and chlorophyll synthesis in bacteria. Ann N Y Acad Sci. 1975 Apr 15;244:334–347. doi: 10.1111/j.1749-6632.1975.tb41540.x. [DOI] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Segen B. J., Gibson K. D. Deficiencies of chromatophore proteins in some mutants of Rhodopseudomonas spheroides with altered carotenoids. J Bacteriol. 1971 Mar;105(3):701–709. doi: 10.1128/jb.105.3.701-709.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solioz M., Yen H. C., Marris B. Release and uptake of gene transfer agent by Rhodopseudomonas capsulata. J Bacteriol. 1975 Aug;123(2):651–657. doi: 10.1128/jb.123.2.651-657.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]


