Abstract
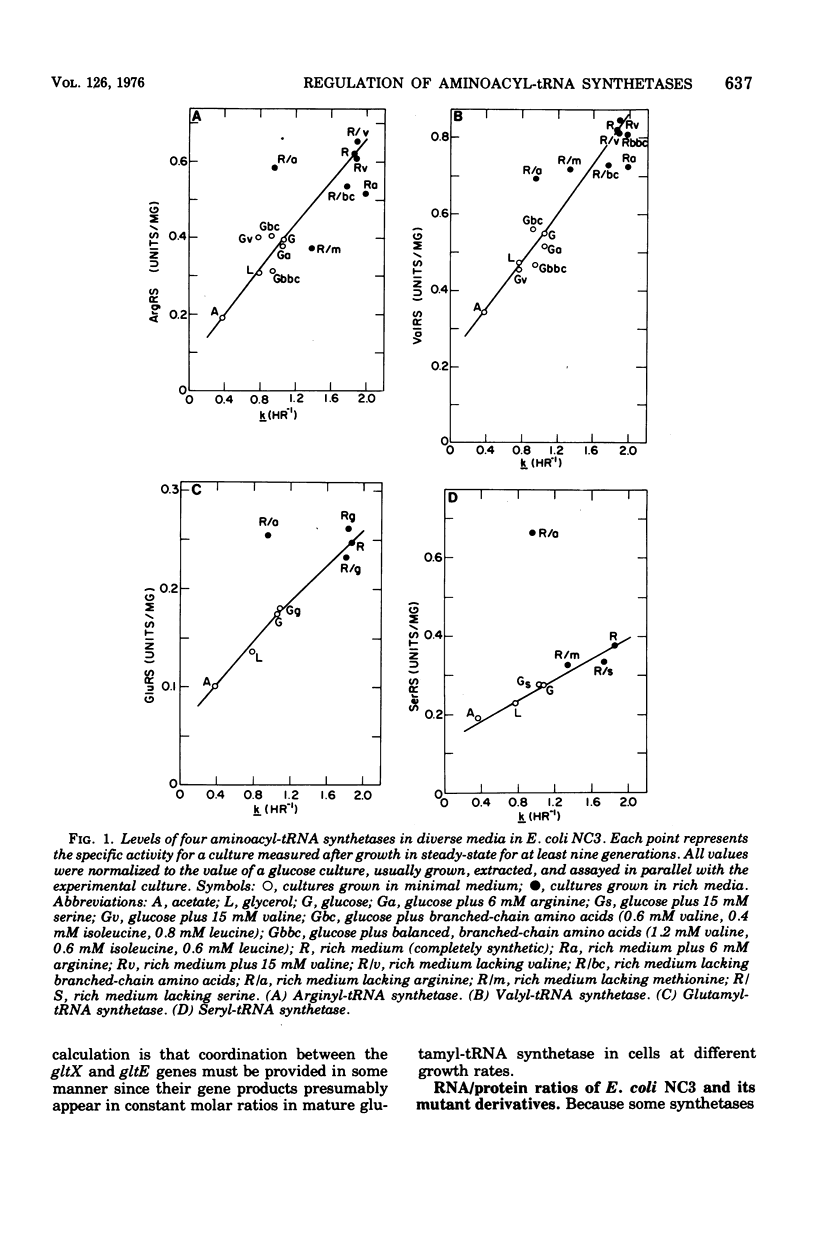
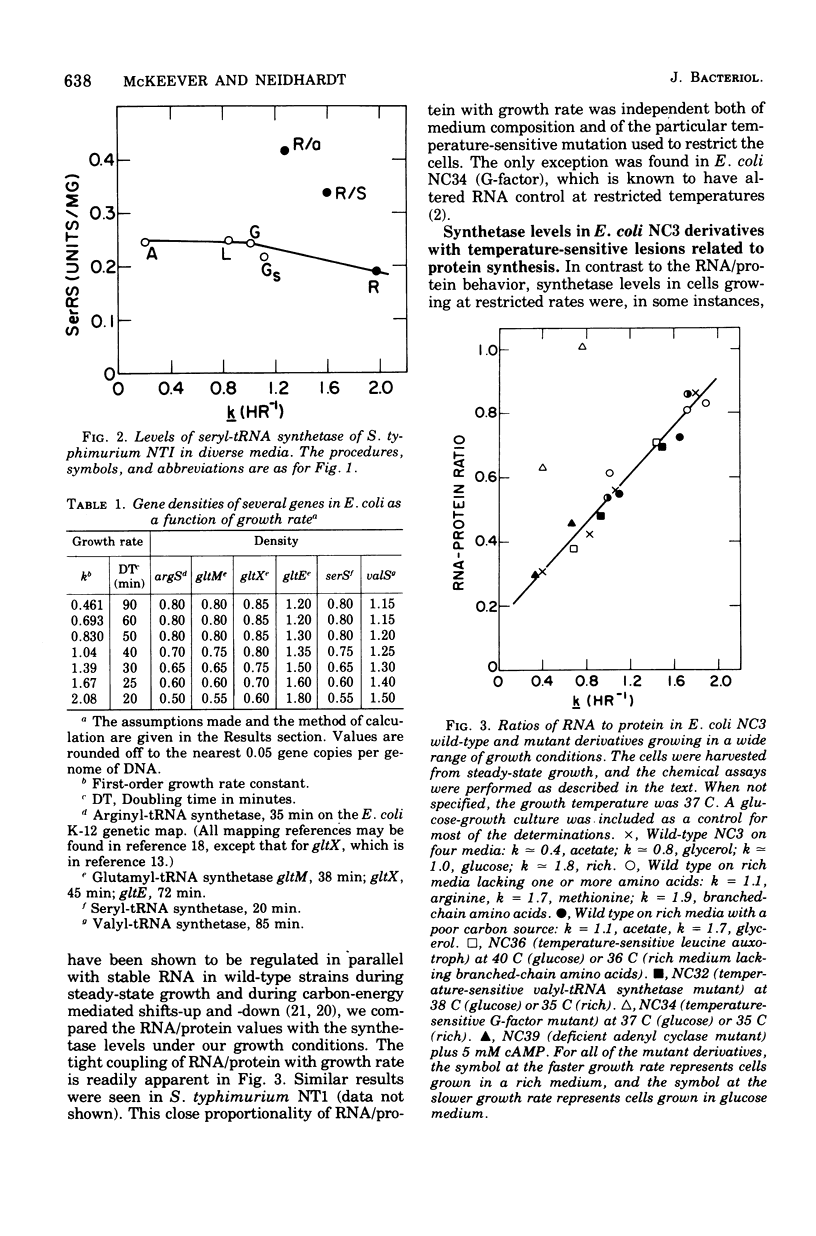
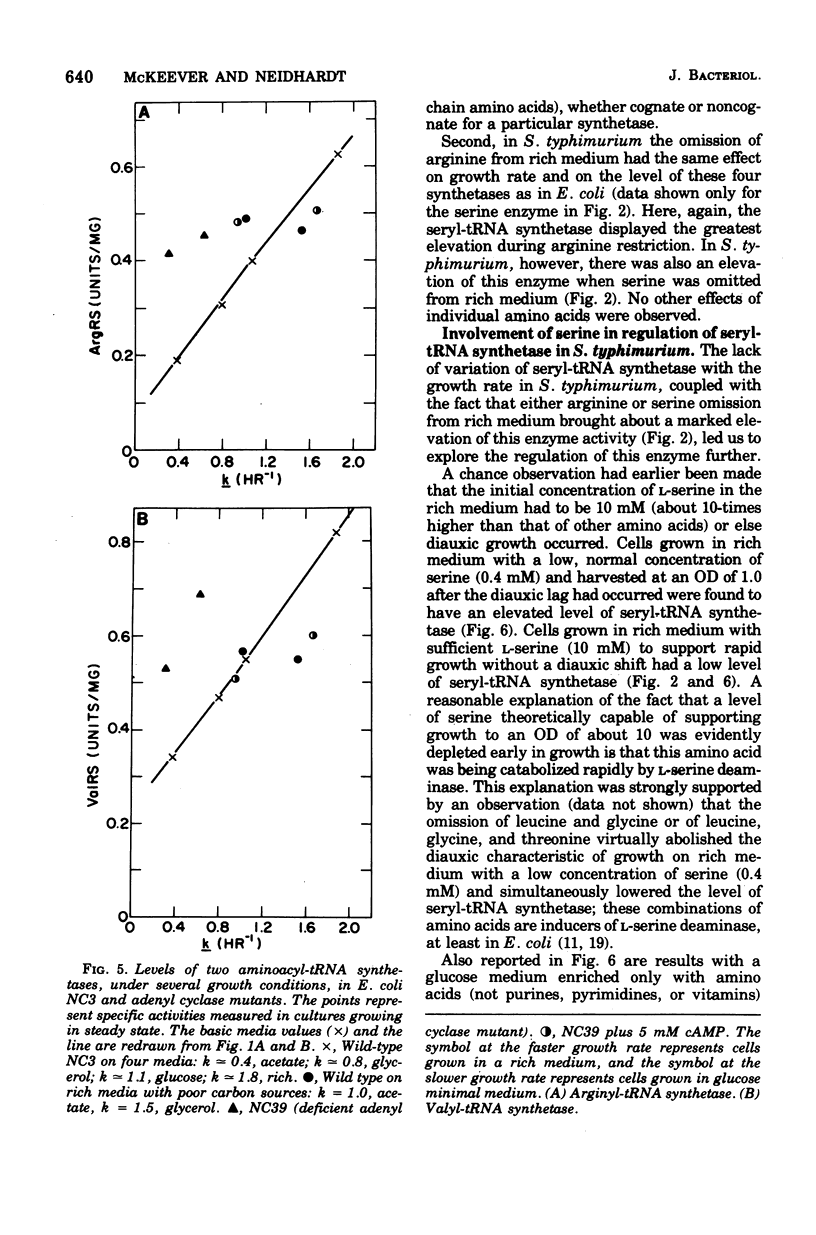
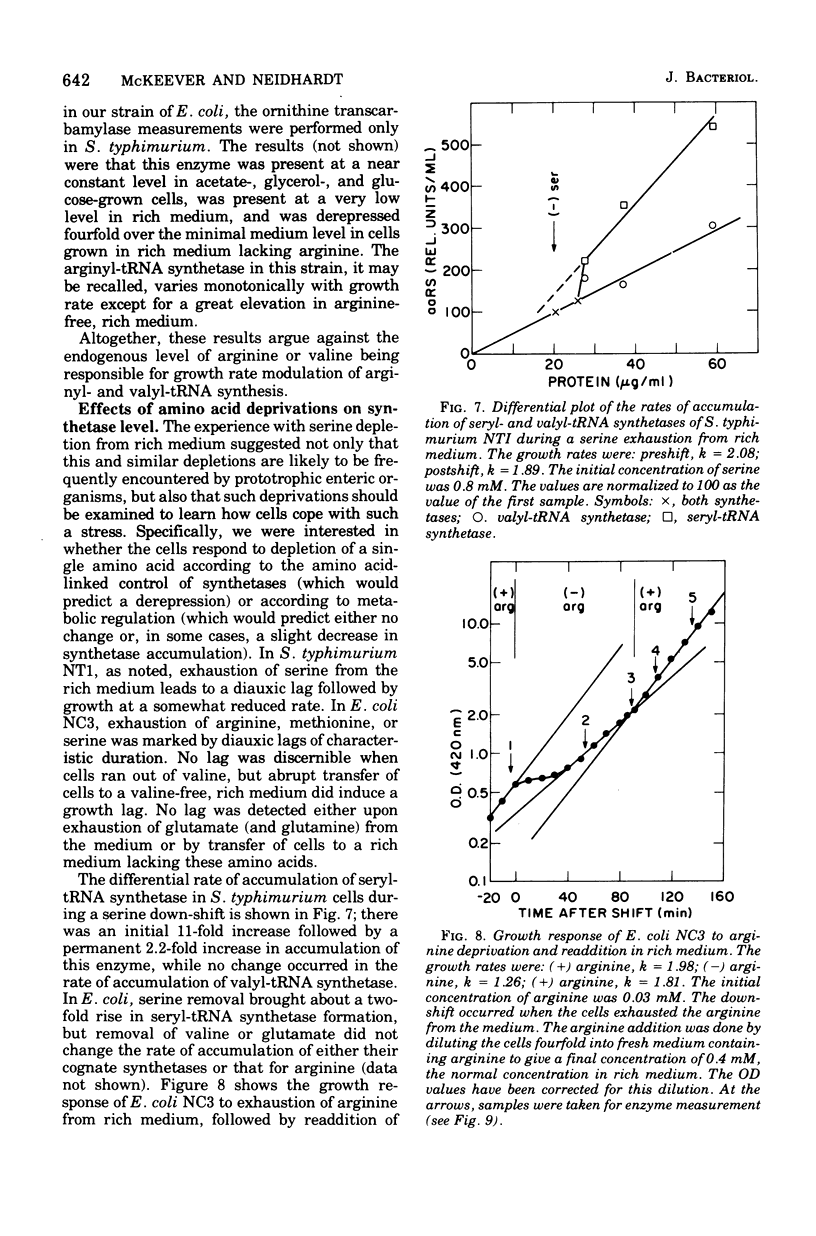
The specific activities of arginyl- glutamyl- seryl-, and valyl-transfer ribonucleic acid (tRNA) synthetases were measured in the wild-type and mutant strains of Salmonella typhimurium LT2 and Escherichia coli B/r. In media restricted only by carbon and energy source availability, the specific activities of all four enzymes were proportional to the growth rate, with the exception of seryl-tRNA synthetase in S. typhimurium, which remained essentially constant. Structural gene densities were calculated for these four enzymes and were found not to account for the variation of specific activity with growth rate.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Neidhardt F. C. Unusual valyl-transfer ribonucleic acid synthetase mutant of Escherichia coli. J Bacteriol. 1972 Jan;109(1):307–314. doi: 10.1128/jb.109.1.307-314.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherly A. G. Temperature-sensitive relaxed Phenotype in a stringent strain of Escherichia coli. J Bacteriol. 1973 Jan;113(1):178–182. doi: 10.1128/jb.113.1.178-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- Cassio D., Mathien Y., Waller J. P. Enhanced level and metabolic regulation of methionyl-transfer ribonucleic acid synthetase in different strains of Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):580–588. doi: 10.1128/jb.123.2.580-588.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Datta P. Purification and feedback control of threonine deaminase activity of Rhodopseudomonas spheroides. J Biol Chem. 1966 Dec 25;241(24):5836–5844. [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Differential rate of ribosomal protein synthesis in Escherichia coli B/r. J Mol Biol. 1974 Apr 15;84(3):407–422. doi: 10.1016/0022-2836(74)90449-5. [DOI] [PubMed] [Google Scholar]
- Fujisawa T., Eisenstark A. Bi-directional chromosomal replication in Salmonella typhimurium. J Bacteriol. 1973 Jul;115(1):168–176. doi: 10.1128/jb.115.1.168-176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. Regulation of the in vivo synthesis of the polypeptide chain elongation factors in Escherichia coli. Biochemistry. 1970 Feb 17;9(4):912–917. doi: 10.1021/bi00806a028. [DOI] [PubMed] [Google Scholar]
- Isenberg S., Newman E. B. Studies on L-serine deaminase in Escherichia coli K-12. J Bacteriol. 1974 Apr;118(1):53–58. doi: 10.1128/jb.118.1.53-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapointe J., Delcuve G. Thermosensitive mutants of Escherichia coli K-12 altered in the catalytic Subunit and in a Regulatory factor of the glutamy-transfer ribonucleic acid synthetase. J Bacteriol. 1975 May;122(2):352–358. doi: 10.1128/jb.122.2.352-358.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Flashner M., Mckeever W. G., Neidhardt F. C. Metabolic regulation of the arginyl and valyl transfer ribonucleic acid synthetases in bacteria. J Biol Chem. 1974 Feb 25;249(4):1044–1053. [PubMed] [Google Scholar]
- Parker J., Neidhardt F. C. Metabolic regulation of aminoacyl-tRNA synthetase formation in bacteria. Biochem Biophys Res Commun. 1972 Oct 17;49(2):495–501. doi: 10.1016/0006-291x(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Skjold A. C., Juarez H., Hedgcoth C. Relationships among deoxyribonucleic acid, ribonucleic acid, and specific transfer ribonucleic acids in Escherichia coli 15T - at various growth rates. J Bacteriol. 1973 Jul;115(1):177–187. doi: 10.1128/jb.115.1.177-187.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


