Abstract
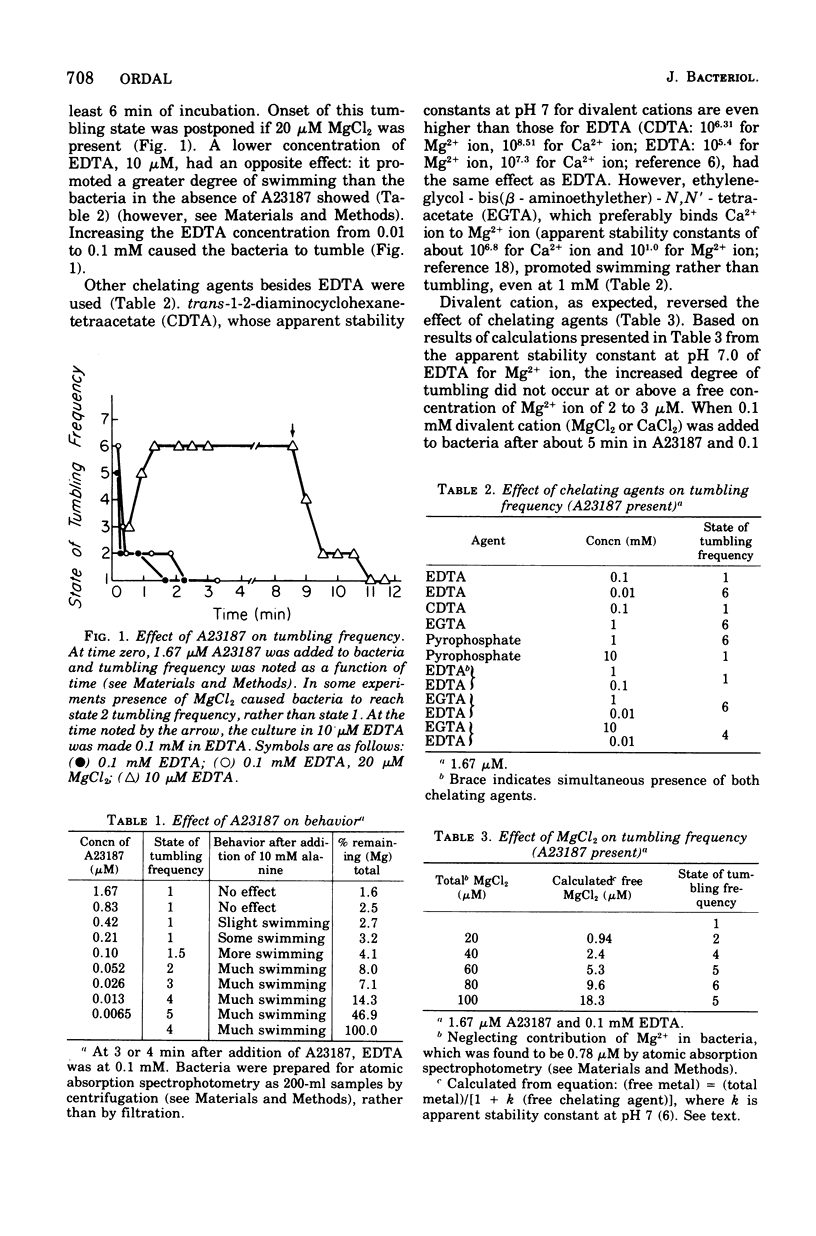
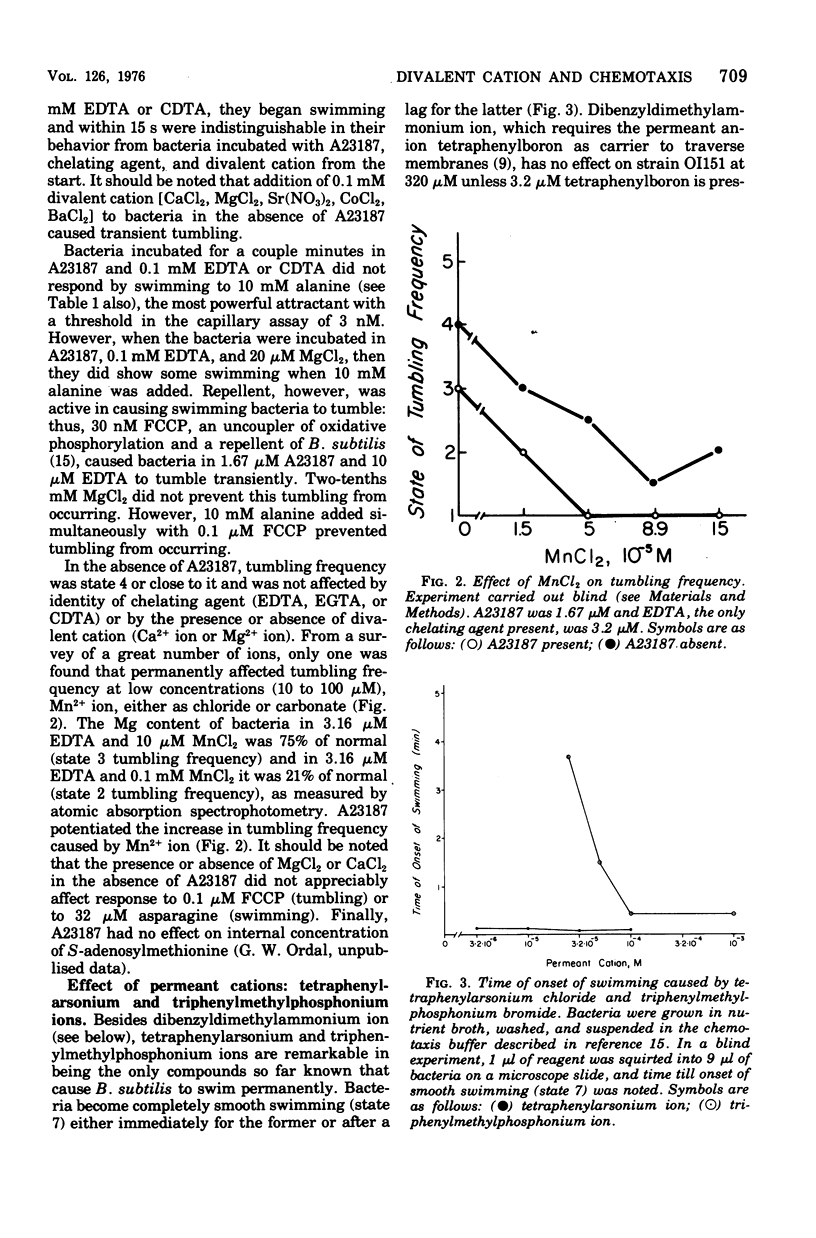
Chemotaxis is migration of organisms to higher concentrations of attractant or lower concentrations of repellent. Understanding the switch than controls whether the flagella rotate counterclockwise for swimming or clockwise for tumbling (thrashing about without making much forward progress) is central to understanding chemotaxis of peritrichous bacteria, since chemotaxis results from selective suppression of tumbles. Depletion of divalent cation by chelating agents in the presence of A23187, an ionophore that conveys divalent cation across membrane, causes incessant tumbling in Bacillus subtilis. Small additions of MgCl2 prevent this tumbling. In this tumbling condition, the bacteria which normally swim extensively when given attractant, do not respond even to 10 mM alanine, a strong attractant. MnCl2, by contrast to others potentiated by the ionophore. Permanent cations, including tetraphenylarsonium ion and triphenylmethylphosphonium ion, cause permanent swimming, even in the presence of A23187 and chelating agents. We propose that divalent cation, probably Mg2+ ion, binds to the switch to cause swimming and that, in the absence of divalent cation at the switch, the bacterium tumbles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Dahl M. M. A method for measuring the motility of bacteria and for comparing random and non-random motility. J Gen Microbiol. 1967 Feb;46(2):161–173. doi: 10.1099/00221287-46-2-161. [DOI] [PubMed] [Google Scholar]
- Armstrong J. B. An S-adenosylmethionine requirement for chemotaxis in Escherichia coli. Can J Microbiol. 1972 Nov;18(11):1695–1701. doi: 10.1139/m72-263. [DOI] [PubMed] [Google Scholar]
- Aswad D. W., Koshland D. E., Jr Evidence for an S-adenosylmethionine requirement in the chemotactic behavior of Salmonella typhimurium. J Mol Biol. 1975 Sep 15;97(2):207–223. doi: 10.1016/s0022-2836(75)80035-0. [DOI] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Role of methionine in bacterial chemotaxis. J Bacteriol. 1974 May;118(2):640–645. doi: 10.1128/jb.118.2.640-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. I. The membrane potential. J Membr Biol. 1972;8(1):27–44. doi: 10.1007/BF01868093. [DOI] [PubMed] [Google Scholar]
- Kort E. N., Goy M. F., Larsen S. H., Adler J. Methylation of a membrane protein involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3939–3943. doi: 10.1073/pnas.72.10.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Naitoh Y., Eckert R. Ionic mechanisms controlling behavioral responses of paramecium to mechanical stimulation. Science. 1969 May 23;164(3882):963–965. doi: 10.1126/science.164.3882.963. [DOI] [PubMed] [Google Scholar]
- Ordal G. W. Effect of methionine on chemotaxis by Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1005–1012. doi: 10.1128/jb.125.3.1005-1012.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotactic repellents of Bacillus subtilis. J Mol Biol. 1976 Jan 5;100(1):103–108. doi: 10.1016/s0022-2836(76)80037-x. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotaxis away from uncouplers of oxidative phosphorylation in Bacillus subtilis. Science. 1975 Sep 5;189(4205):802–805. doi: 10.1126/science.808854. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Scribner H., Eisenstadt E., Silver S. Magnesium transport in Bacillus subtilis W23 during growth and sporulation. J Bacteriol. 1974 Mar;117(3):1224–1230. doi: 10.1128/jb.117.3.1224-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. S., Kort E. N., Larsen S. H., Ordal G. W., Reader R. W., Adler J. Role of methionine in bacterial chemotaxis: requirement for tumbling and involvement in information processing. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4640–4644. doi: 10.1073/pnas.72.11.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]


