Abstract
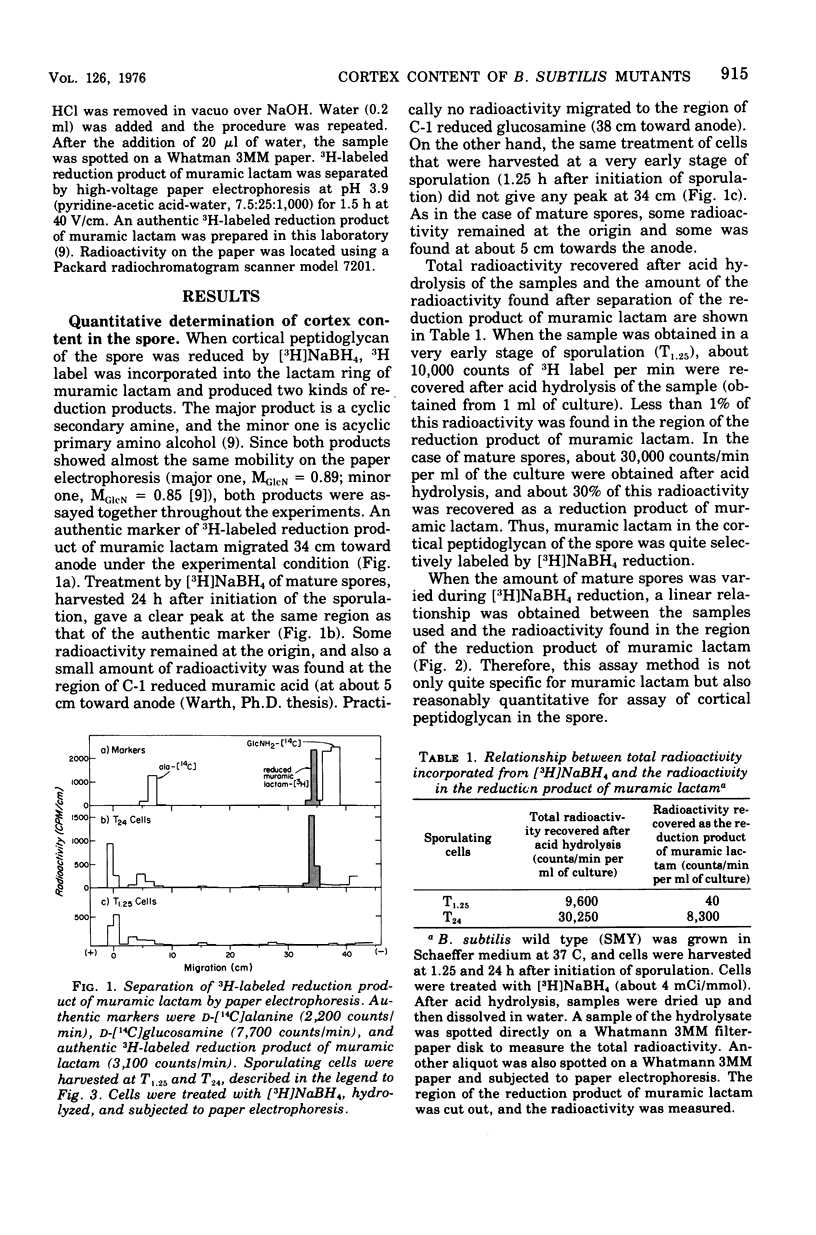
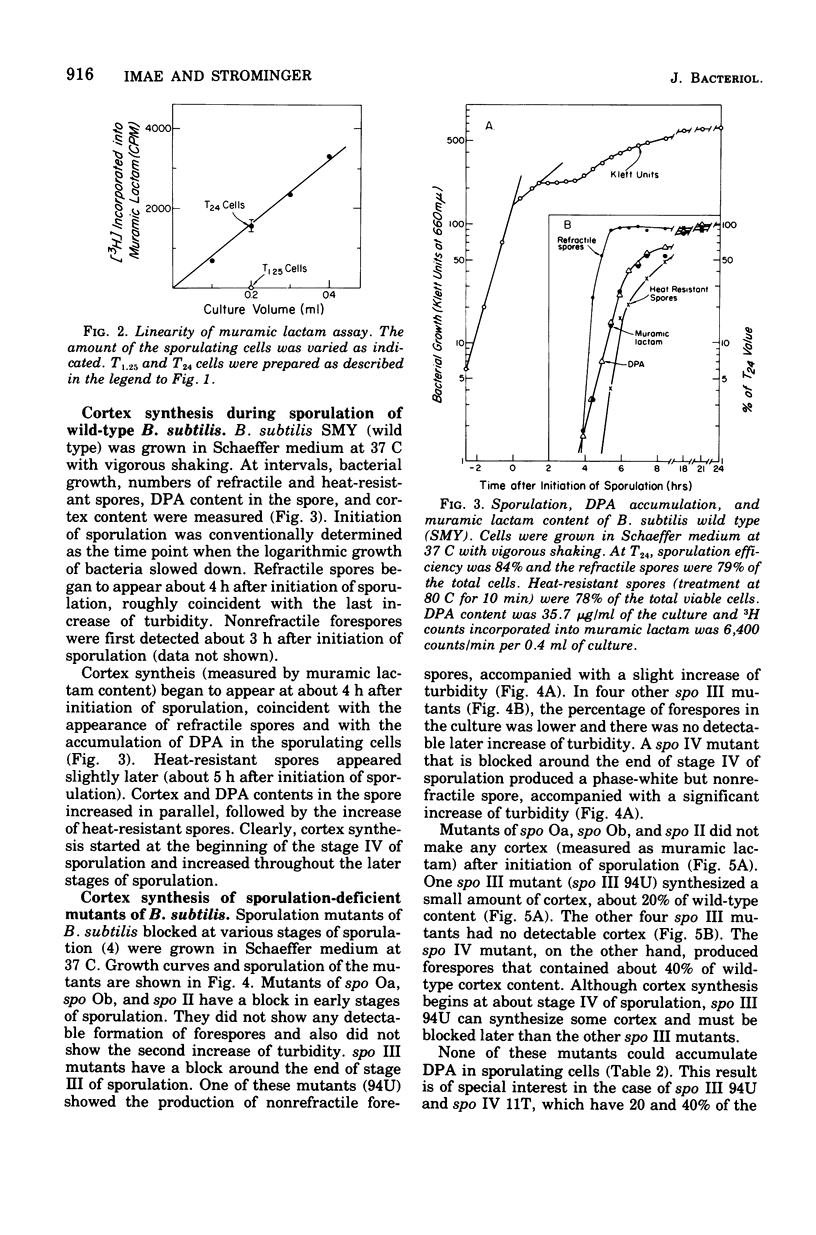
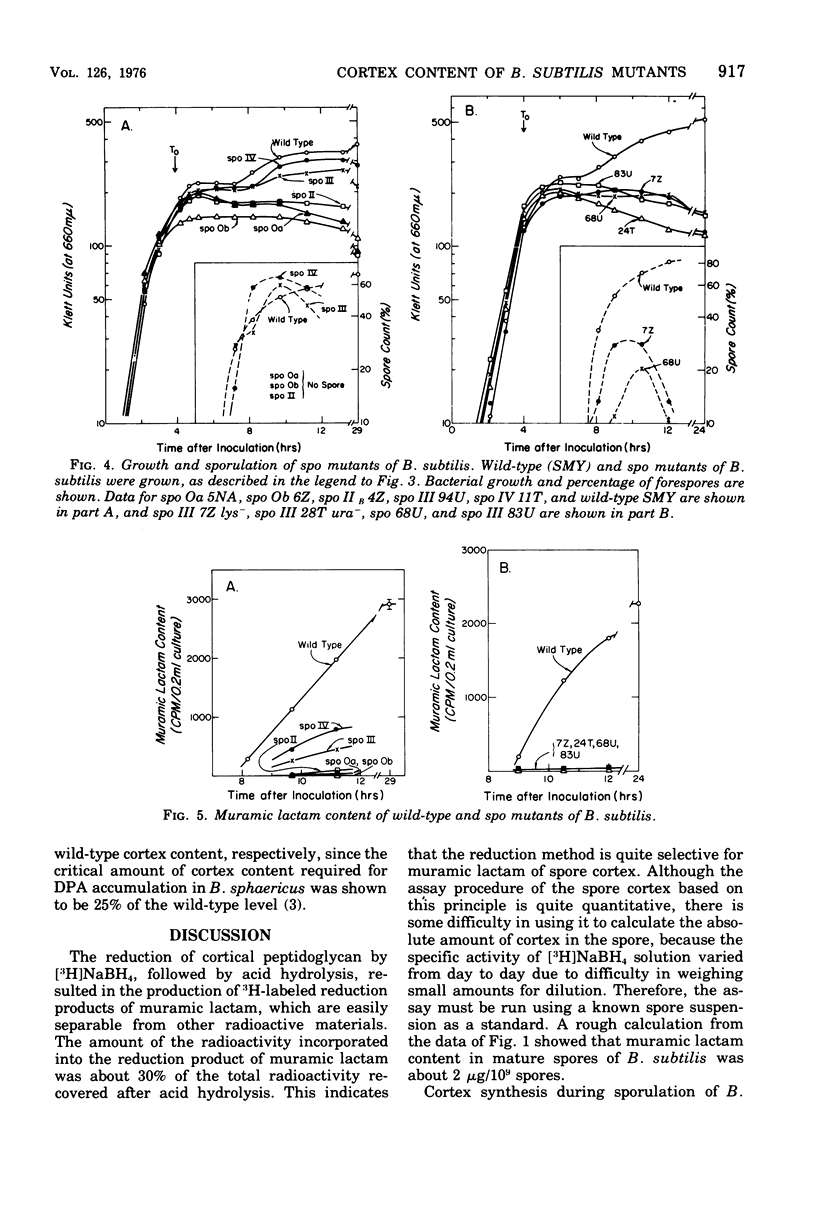
A method for the measurement of muramic lactam, which is specifically located in the cortical peptidoglycan of bacterial spores, was developed as a quantitative assay method for spore cortex content. During sporulation of Bacillus subtilis 168, muramic lactam (i.e., spore cortex) began to appear at state IV of sporulation and continued to increase over most of the late stages of sporulation. Spore cortex contents of various spo mutants of B. subitils were surveyed. Cortex was not detected in mutants in which sporulation was blocked earlier than stage II sporulation. Spores of spo IV mutant had about 40% of the cortex content of the wild-type spores. One spo III mutant had a low amount of cortex, but four others had none.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Imae Y., Strominger J. L. Relationship between cortex content and properties of Bacillus sphaericus spores. J Bacteriol. 1976 May;126(2):907–913. doi: 10.1128/jb.126.2.907-913.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionesco H., Michel J., Cami B., Schaeffer P. Symposium on bacterial spores: II. Genetics of sporulation in Bacillus subtilis Marburg. J Appl Bacteriol. 1970 Mar;33(1):13–24. doi: 10.1111/j.1365-2672.1970.tb05230.x. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Lewis J. C., Snell N. S., Burr H. K. Water Permeability of Bacterial Spores and the Concept of a Contractile Cortex. Science. 1960 Aug 26;132(3426):544–545. doi: 10.1126/science.132.3426.544. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry. 1972 Apr 11;11(8):1389–1396. doi: 10.1021/bi00758a010. [DOI] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan of bacterial spores: occurrence of the lactam of muramic acid. Proc Natl Acad Sci U S A. 1969 Oct;64(2):528–535. doi: 10.1073/pnas.64.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickus G. G., Warth A. D., Strominger J. L. Appearance of muramic lactam during cortex synthesis in sporulating cultures of Bacillus cereus and Bacillus megaterium. J Bacteriol. 1972 Aug;111(2):625–627. doi: 10.1128/jb.111.2.625-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


