Abstract
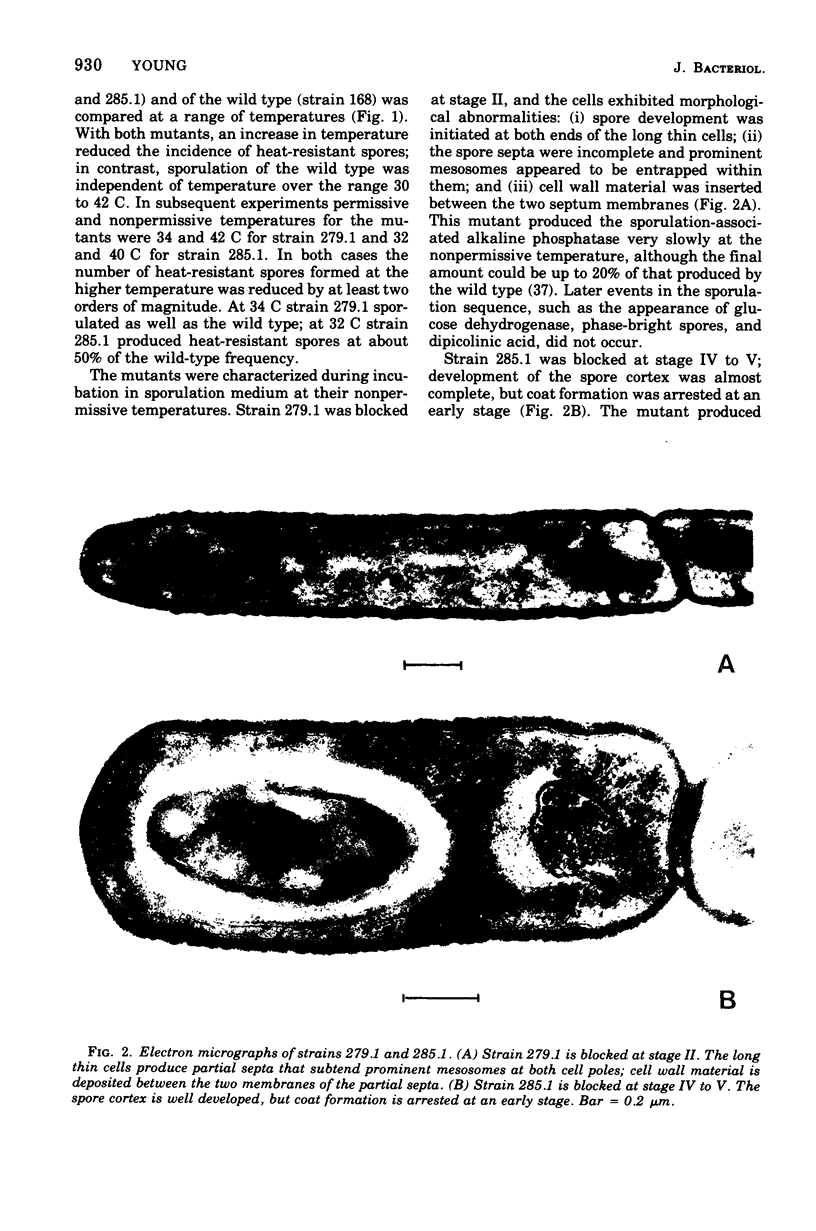
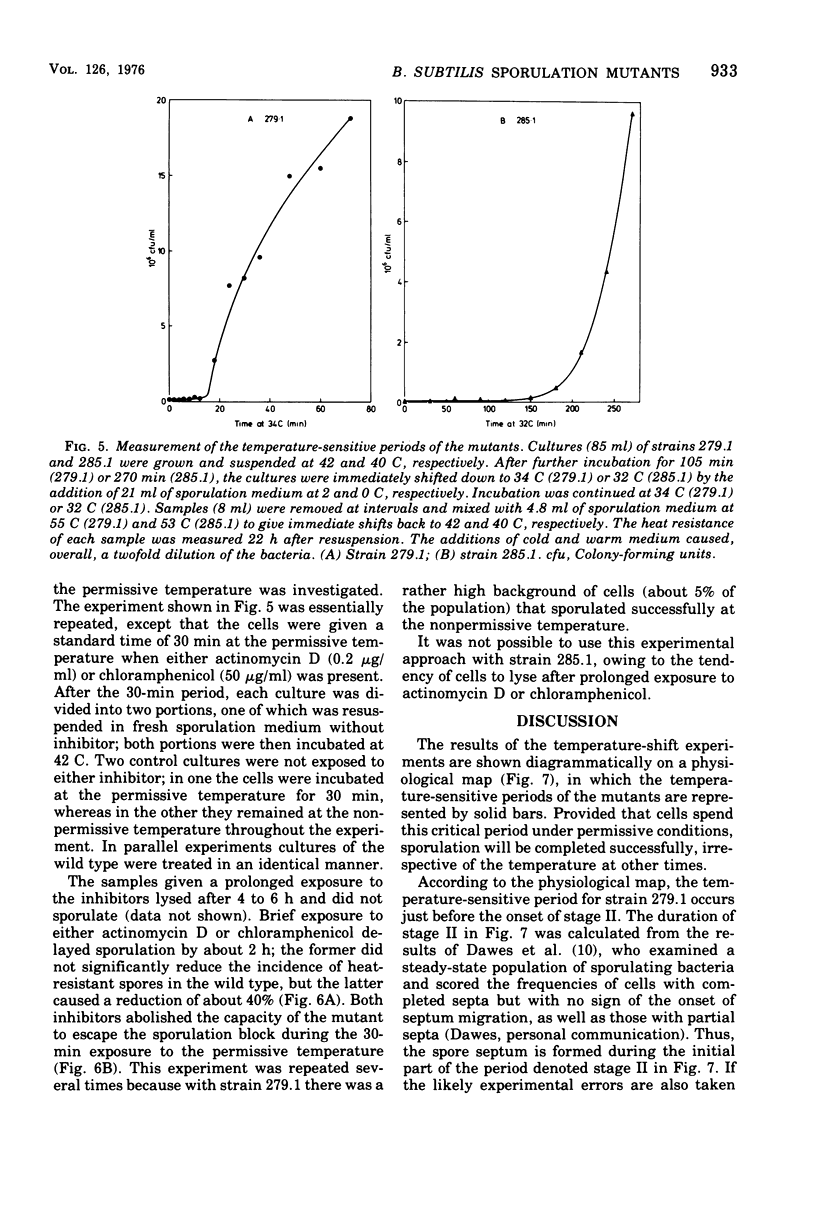
Two temperature-sensitive sporulation mutants have been characterized. One mutant, which is blocked at stage II, has a short temperature-sensitive period that occurs at about the time when the spore septum is formed. Cells can escape the sporulation block, if incubated for a short period at the permissive temperature, but are prevented from doing so by inhibitors of transcription and translation; this suggests that the product of the defective gene is a protein and that the messenger ribonucleic acid which codes for this protein is short-lived. The other mutant is blocked at stage IV to V and has a long temperature-sensitive period that starts during stage III and precedes the stage at which the mutational defect is phenotypically expressed. The behavior of this mutant in temperature-shift experiments suggests that synthesis of the product of the defective gene commences long before it assumes its physiological function.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Fitz-James P. C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968 Apr 14;33(1):199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- Balassa G. Synthèse et fonction des ARN messagers au cours de la sporulation de Bacillus subtilis. Ann Inst Pasteur (Paris) 1966 Feb;110(2):175–191. [PubMed] [Google Scholar]
- Balassa G. The genetic control of spore formation in bacilli. Curr Top Microbiol Immunol. 1971;56:99–192. doi: 10.1007/978-3-642-65241-7_4. [DOI] [PubMed] [Google Scholar]
- Beckman D., Cooper S. Temperature-sensitive nonsense mutations in essential genes of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1336–1342. doi: 10.1128/jb.116.3.1336-1342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T., Hastings J. W. Temperature-sensitive mutants of bioluminescent bacteria. Proc Natl Acad Sci U S A. 1971 Feb;68(2):500–504. doi: 10.1073/pnas.68.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G., Mandelstam J. Use of constructed double mutants for determining the temporal order of expression of sporulation genes in Bacillus subtilis. J Bacteriol. 1973 Jun;114(3):1254–1263. doi: 10.1128/jb.114.3.1254-1263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G. Sporulation in Bacillus subtilis. Characterization of oligosporogenous mutants and comparison of their phenotypes with those of asporogenous mutants. J Gen Microbiol. 1972 Jun;71(1):1–15. doi: 10.1099/00221287-71-1-1. [DOI] [PubMed] [Google Scholar]
- Dancer B. N., Mandelstam J. Production and possible function of serine protease during sporulation of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):406–410. doi: 10.1128/jb.121.2.406-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes I. W., Kay D., Mandelstam J. Sporulation in Bacillus subtilis. Establishment of a time scale for the morphological events. J Gen Microbiol. 1969 May;56(2):171–179. doi: 10.1099/00221287-56-2-171. [DOI] [PubMed] [Google Scholar]
- EDGAR R. S., DENHARDT G. H., EPSTEIN R. H. A COMPARATIVE GENETIC STUDY OF CONDITIONAL LETHAL MUTATIONS OF BACTERIOPHAGE T4D. Genetics. 1964 Apr;49:635–648. doi: 10.1093/genetics/49.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Conditional mutants of meiosis in yeast. J Bacteriol. 1970 Oct;104(1):202–210. doi: 10.1128/jb.104.1.202-210.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D. Sporulation in Bacillus subtilis 168. Control of synthesis of alkaline phosphatase. J Gen Microbiol. 1974 Jun;82(2):363–369. doi: 10.1099/00221287-82-2-363. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Jockusch H. Relations between temperature sensitivity, amino acid replacements, and quaternary structure of mutant proteins. Biochem Biophys Res Commun. 1966 Aug 23;24(4):577–583. doi: 10.1016/0006-291x(66)90360-3. [DOI] [PubMed] [Google Scholar]
- Kay D., Warren S. C. Sporulation in Bacillus subtilis. Morphological changes. Biochem J. 1968 Oct;109(5):819–824. doi: 10.1042/bj1090819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J. An RNA polymerase mutation causing temperature-sensitive sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1179–1183. doi: 10.1073/pnas.70.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. Further studies on the stability of sporulation messenger ribonucleic acid in Bacillus subtilis. J Biol Chem. 1974 Dec 25;249(24):7808–7812. [PubMed] [Google Scholar]
- Piggot P. J. Mapping of asporogenous mutations of Bacillus subtilis: a minimum estimate of the number of sporeulation operons. J Bacteriol. 1973 Jun;114(3):1241–1253. doi: 10.1128/jb.114.3.1241-1253.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- Rasse-Messenguy F., Fink G. R. Temperature-sensitive nonsense suppressors in yeast. Genetics. 1973 Nov;75(3):459–464. doi: 10.1093/genetics/75.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- SADLER J. R., NOVICK A. THE PROPERTIES OF REPRESSOR AND THE KINETICS OF ITS ACTION. J Mol Biol. 1965 Jun;12:305–327. doi: 10.1016/s0022-2836(65)80255-8. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki D. T. Temperature-sensitive mutations in Drosophila melanogaster. Science. 1970 Nov 13;170(3959):695–706. doi: 10.1126/science.170.3959.695. [DOI] [PubMed] [Google Scholar]
- Szulmajster J., Bonamy C., Laporte J. Isolation and properties of a temperature-sensitive sporulation mutant of Bacillus subtilis. J Bacteriol. 1970 Mar;101(3):1027–1037. doi: 10.1128/jb.101.3.1027-1037.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Jauregui-Adell J., Wittmann H. G. Die primäre Proteinstruktur Temperatur-sensitiver Mutanten des Tabakmosaikvirus. II. Chemisch induzierte Mutanten. Z Naturforsch B. 1965 Dec;20(12):1235–1249. [PubMed] [Google Scholar]
- Wittmann H. G., Wittmann-Liebold B., Jauregui-Adell J. Die primäre Proteinstruktur Temperatur-sensitiver Mutanten des Tabakmosaikvirus I. Spontanmutanten. Z Naturforsch B. 1965 Dec;20(12):1224–1234. [PubMed] [Google Scholar]
- Wood D. A. Sporulation in Bacillus subtilis. Properties and time of synthesis of alkali-soluble protein of the spore coat. Biochem J. 1972 Nov;130(2):505–514. doi: 10.1042/bj1300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. Genetic mapping of sporulation operons in Bacillus subtilis using a thermosensitive sporulation mutant. J Bacteriol. 1975 Jun;122(3):1109–1116. doi: 10.1128/jb.122.3.1109-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



