Abstract
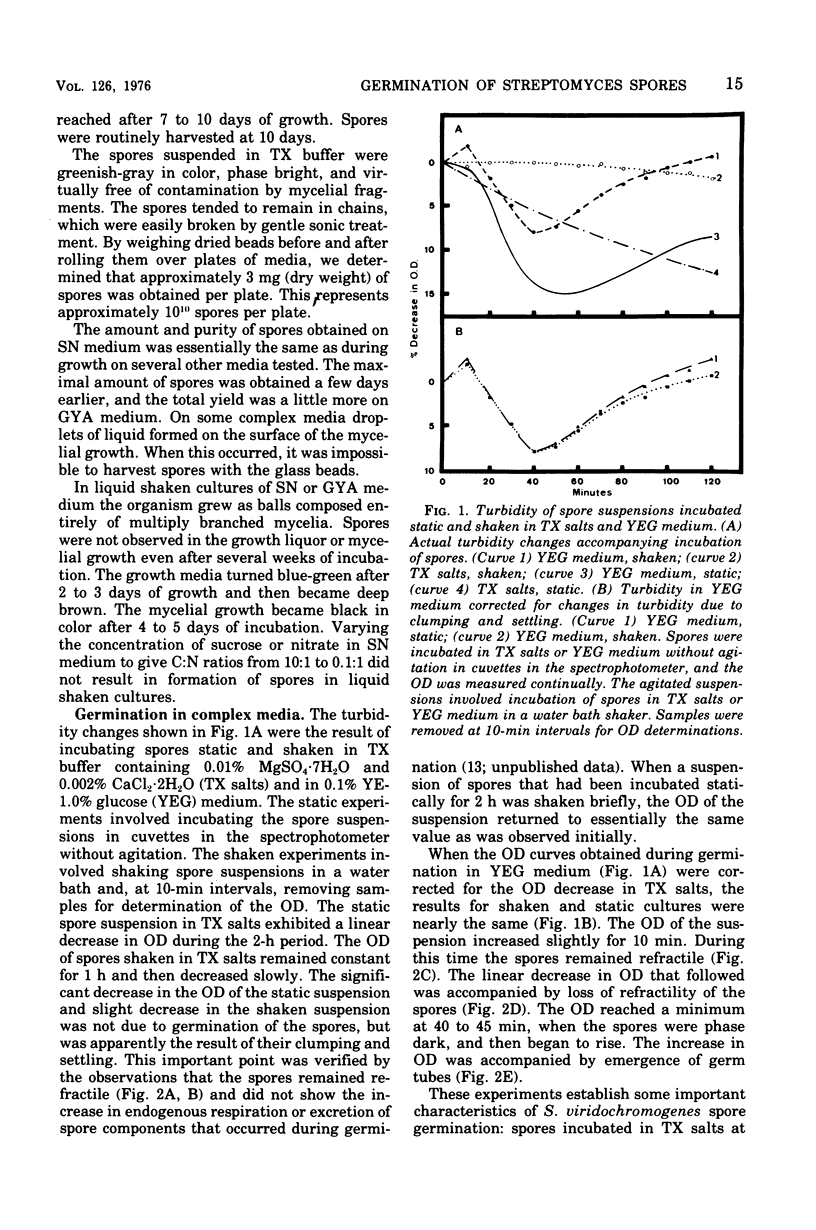
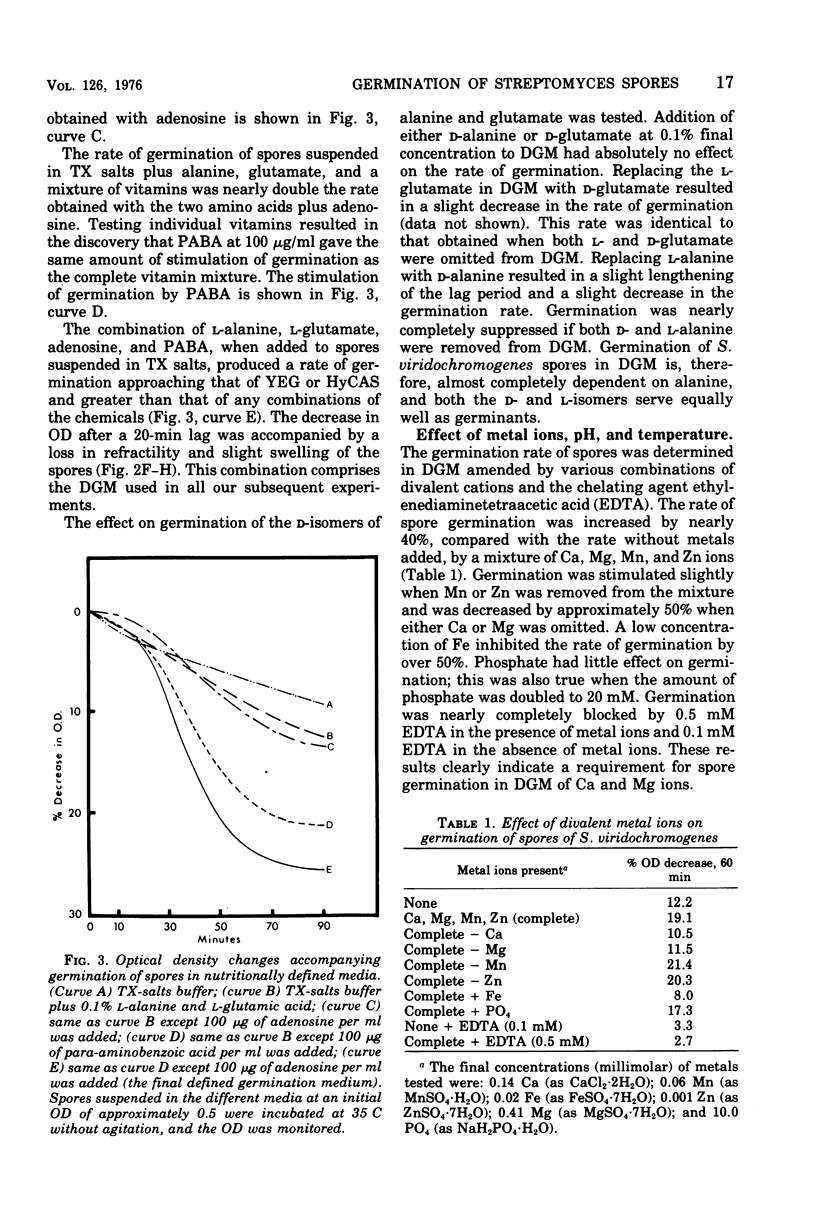
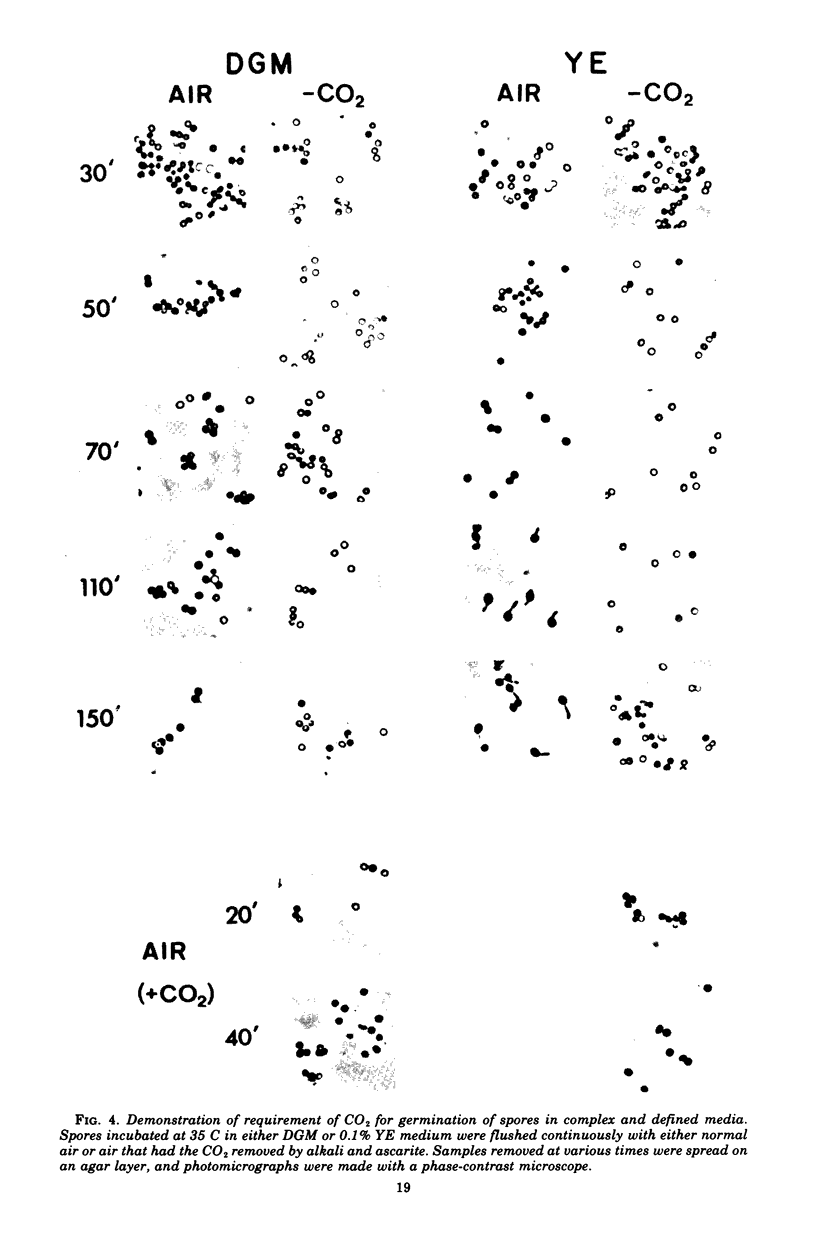
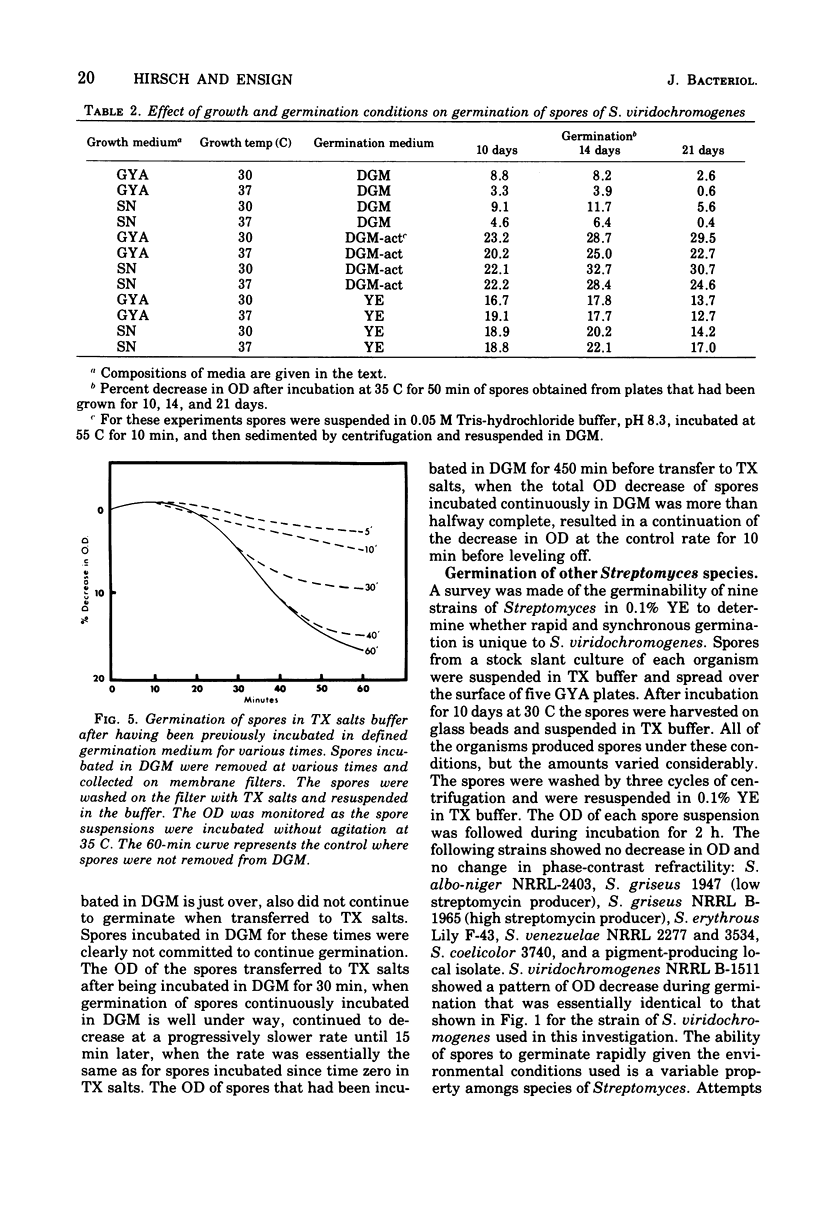
Spores of Streptomyces viridochromogenes were removed from the surface of solid media with glass beads and suspended in a buffer-detergent solution. Addition of yeast extract and glucose resulted in rapid loss of refractility of the spores. Appearance of germ tubes followed. Germination was accompanied by a decrease in the optical density (OD) of the suspension. The OD decrease was used as an assay for germination. A defined germination medium (DGM) comprised of L-alanine, L-glutamic acid, adenosine, para-aminobenzoic acid, and calcium and magnesium ions provided a germination rate nearly equal to that of complex media. The germination rate was essentially the same if D-alanine and D-glutamate replaced the L-isomers. The optimum pH and temperature for germination were 7.0 and 35 C. Germination was absolutely dependent on the presence of CO2. Spores harvested after growth for longer periods than the usual time (10 days) became less germinable in DGM. The same was observed for spores grown at 37 C as compared with 30 C. Spores incubated in DGM for various time periods before being transferred to a buffer solution did not continue to germinate. Spores harvested after growth of eight species of Streptomyces did not show a decrease in OD when incubated in yeast extract medium. Another strain of S. viridochromogenes did exhibit an OD decrease in the medium. Comparative properties of spores of streptomycetes, fungi, and bacilli are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell R. W., Cross T. Germination of actinomycete spores. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:197–207. [PubMed] [Google Scholar]
- Bradley S. G., Ritzi D. Composition and ultrastructure of Streptomyces venezuelae. J Bacteriol. 1968 Jun;95(6):2358–2364. doi: 10.1128/jb.95.6.2358-2364.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong P. J., McCoy E. Qualitative analyses of vegetative cell walls and spore walls of some representative species of Streptomyces. Can J Microbiol. 1966 Oct;12(5):985–994. doi: 10.1139/m66-133. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., WOLFE R. S. NUTRITIONAL CONTROL OF MORPHOGENESIS IN ARTHROBACTER CRYSTALLOPIETES. J Bacteriol. 1964 Apr;87:924–932. doi: 10.1128/jb.87.4.924-932.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D. General consideration and implications of the Actinomycetales. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:1–10. [PubMed] [Google Scholar]
- HALMANN M., KEYNAN A. Stages in germination of spores of Bacillus licheniformis. J Bacteriol. 1962 Dec;84:1187–1193. doi: 10.1128/jb.84.6.1187-1193.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPWOOD D. A., GLAUERT A. M. Observations on the chromatinic bodies of Streptomyces coelicolor. J Biophys Biochem Cytol. 1960 Sep;8:257–265. doi: 10.1083/jcb.8.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPWOOD D. A. Phase-contrast observations on Streptomyces coelicolor. J Gen Microbiol. 1960 Feb;22:295–302. doi: 10.1099/00221287-22-1-295. [DOI] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. Interaction of heat, glucose, L-alanine, and potassium nitrate in spore germination of Bacillus megaterium. J Bacteriol. 1961 Feb;81:204–211. doi: 10.1128/jb.81.2.204-211.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen R. J., Ensign J. C. Effect of growth substrates on morphology of Nocardia corallina. Arch Microbiol. 1975 May 5;103(3):209–217. doi: 10.1007/BF00436352. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Silvey J. K. Slide culture observations of two freshwater actinomycetes. Trans Am Microsc Soc. 1966 Jul;85(3):390–398. [PubMed] [Google Scholar]
- Kalakoutskii L. V., Pouzharitskaja L. M. The Streptomyces spore: its distinct features and germinal behaviour. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:155–178. [PubMed] [Google Scholar]
- Kalakutskii L. V., Bobkova E. A. L-valin i prorastanie konidii Actinomyces streptomycini B-6. Dokl Akad Nauk SSSR. 1966 Oct 11;170(5):1192–1194. [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C. Alteration of glucose metabolism of Arthrobacter crystallopoietes by compounds which induce sphere to rod morphogenesis. J Bacteriol. 1969 Feb;97(2):526–534. doi: 10.1128/jb.97.2.526-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth H. Development and organization of the aerial mycelium in Streptomyces coelicolor. J Gen Microbiol. 1970 Jan;60(1):43–50. doi: 10.1099/00221287-60-1-43. [DOI] [PubMed] [Google Scholar]
- Wildermuth H., Hopwood D. A. Septation during sporulation in Streptomyces coelicolor. J Gen Microbiol. 1970 Jan;60(1):51–59. doi: 10.1099/00221287-60-1-51. [DOI] [PubMed] [Google Scholar]