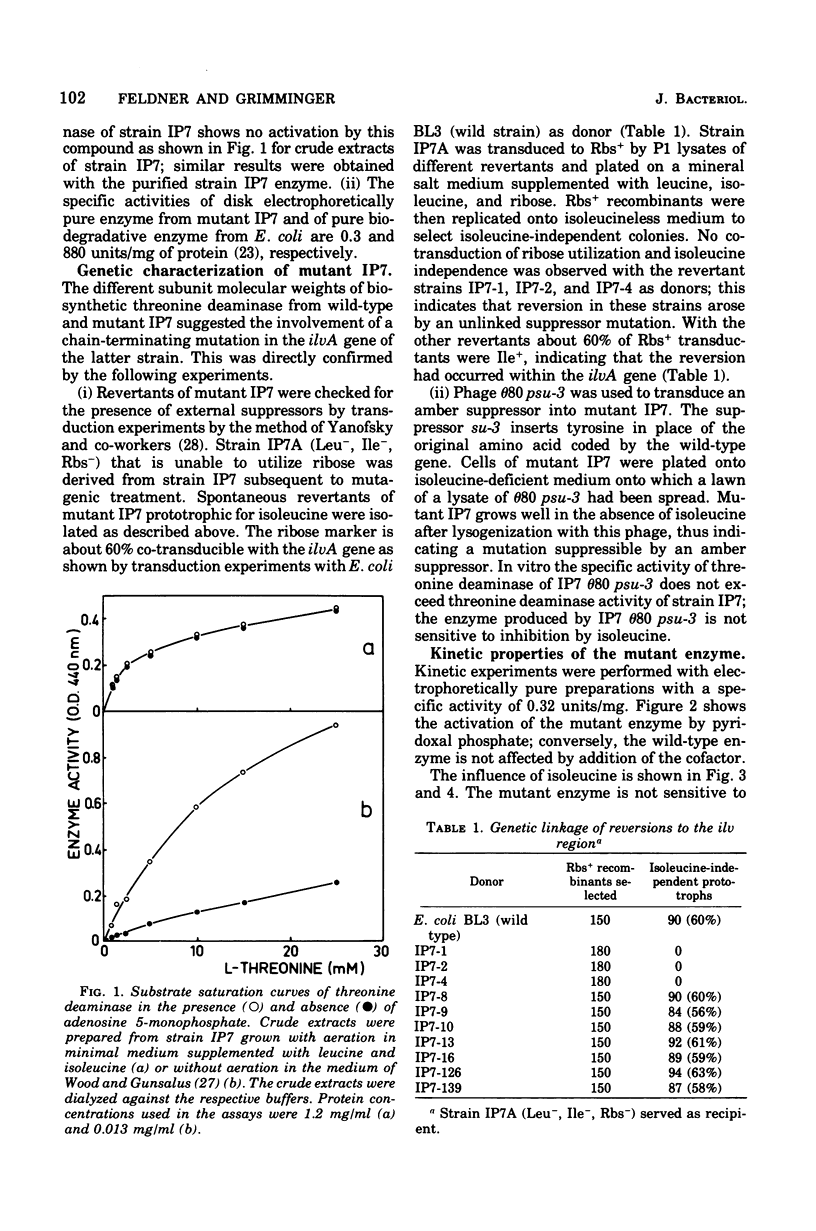
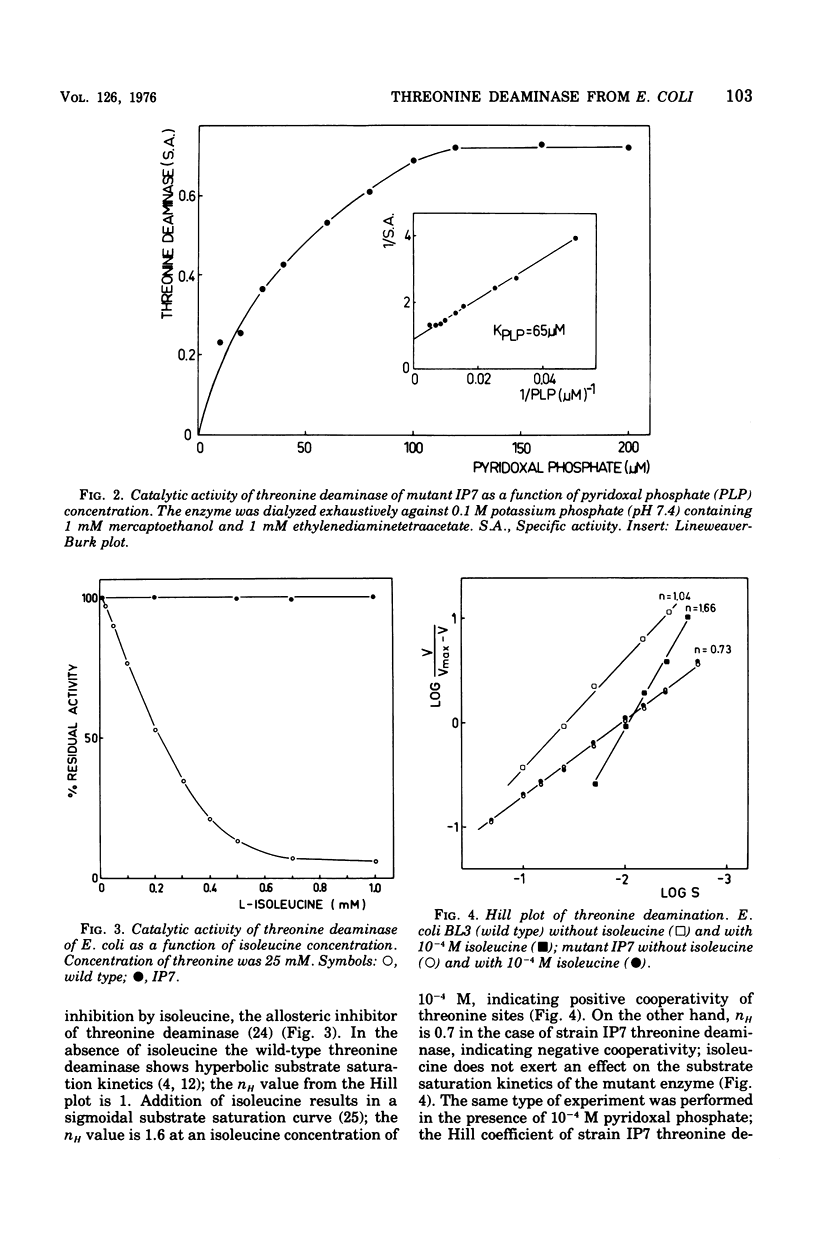
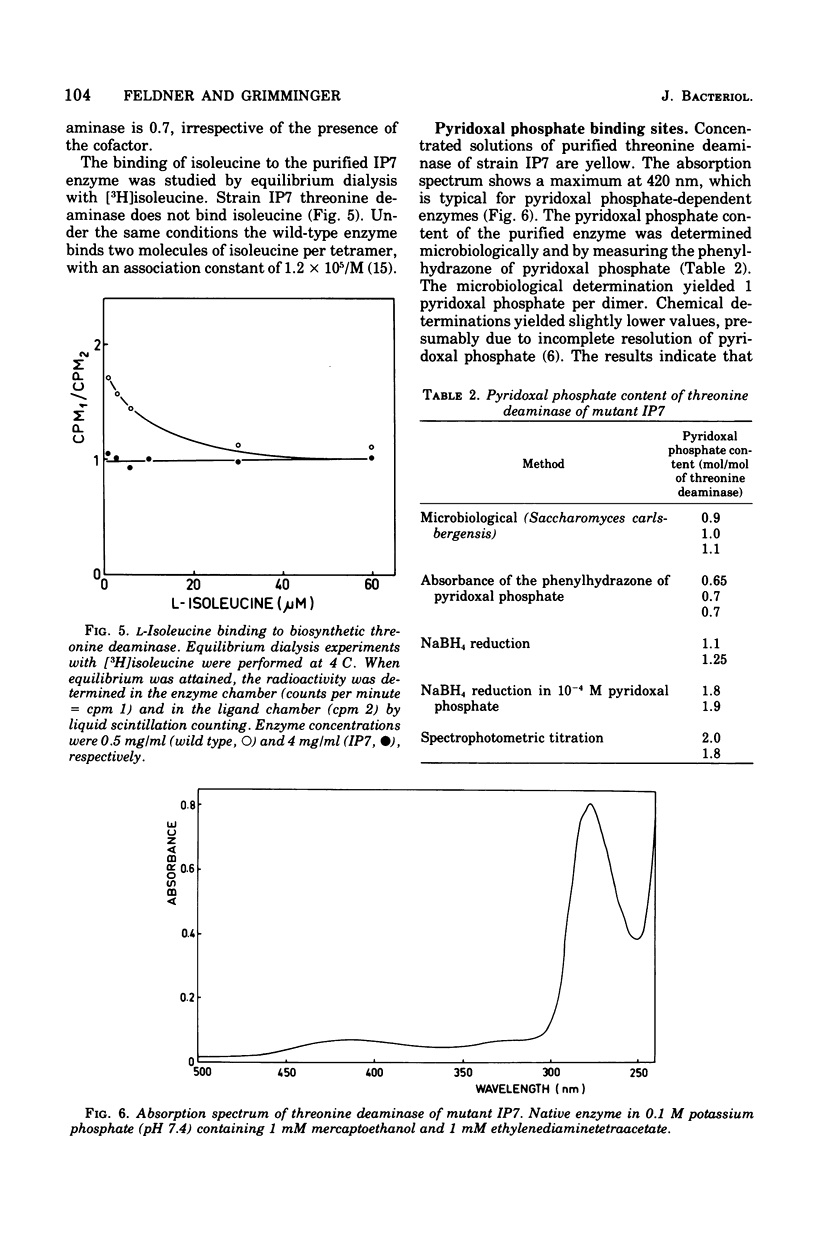
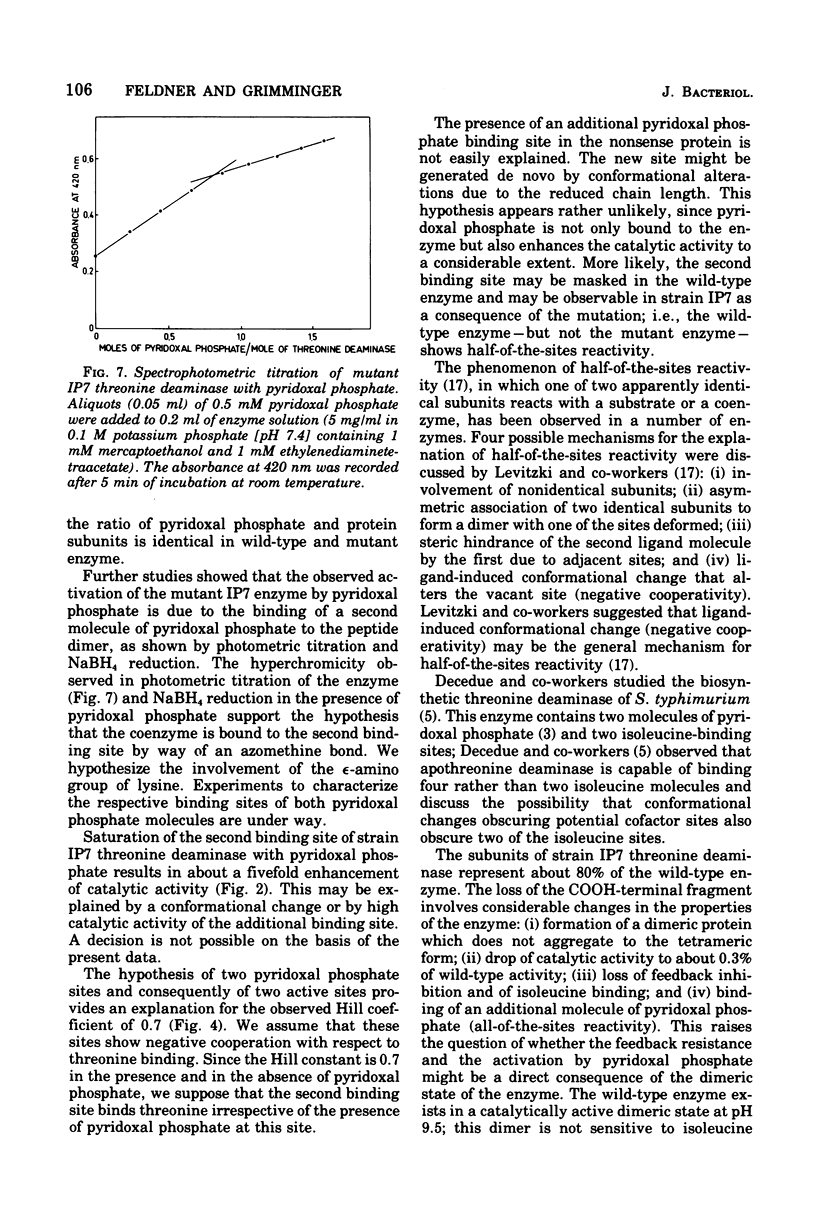
Abstract
The mutant IP7 of Escherichia coli B requires isoleucine or pyridoxine for growth as a consequence of a mutation in the gene coding for biosynthetic threonine deaminase. The mutation of IP7 was shown to be of the nonsense type by the following data: (1) reversion to isoleucine prototrophy involves the formation of external suppression at a high frequency, as shown by transduction experiments; and (ii) the isoleucine requirement is suppressed by lysogenization with a phage carrying the amber suppressor su-3. Cell extracts of the mutant strain contain a low activity of threonine deaminase. The possibility that this activity is biodegradative was ruled out by kinetic experiments. The mutant threonine deaminase was purified to homogeneity by conventional procedures. The enzyme is a dimer of identical subunits of an approximate molecular weight of 43,000 (Grimminger and Feldner, 1974), whereas the wild-type enzyme is a tetramer of 50,000-dalton subunits (Calhoun et al., 1973; Grimminger et al., 1973). The mutant enzyme is not inhibited by isoleucine and does not bind isoleucine, as shown by equilibrium dialysis experiments. Pyridoxal phosphate enhances the maximum catalytic activity of the mutant enzyme by a factor of five, whereas the wild-type enzyme is not affected. In wild-type and mutant threonine deaminase the ratio of protein subunits and bound pyridoxal phosphate is 2:1. The activation of threonine deaminase from strain IP7 is due to a second coenzyme binding site, as shown by (i) spectrophotometric titration of the enzyme with pyridoxal phosphate and by (ii) measurement the pyridoxal phosphate content of the enzyme after sodium borohydride reduction of the protein. The observation of one pyridoxal phosphate binding site per peptide dimer in the wild-type enzyme and of two binding sites per dimer in the mutant strongly suggests that one of the potential sites in the wild-type enzyme is masked by allosteric effects. The factors responsible for the half-of-the-sites reactivity of the coenzyme sites appear to be nonoperative in the mutant protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babul J., Stellwagen E. Measurement of protein concentration with interferences optics. Anal Biochem. 1969 Apr 4;28(1):216–221. doi: 10.1016/0003-2697(69)90172-9. [DOI] [PubMed] [Google Scholar]
- Burns R. O., Zarlengo M. H. Threonine deaminase from Salmonella typhimurium. I. Purification and properties. J Biol Chem. 1968 Jan 10;243(1):178–185. [PubMed] [Google Scholar]
- Calhoun D. H., Rimerman R. A., Hatfield G. W. Threonine deaminase from Escherichia coli. I. Purification and properties. J Biol Chem. 1973 May 25;248(10):3511–3516. [PubMed] [Google Scholar]
- Decedue C. J., Hofler J. G., Burns R. O. Threonine deaminase from Salmonella typhimurium. Relationship between regulatory sites. J Biol Chem. 1975 Feb 25;250(4):1563–1570. [PubMed] [Google Scholar]
- Dupourque D., Newton W. A., Snell E. E. Purification and properties of D-serine dehydrase from Escherichia coli. J Biol Chem. 1966 Mar 10;241(5):1233–1238. [PubMed] [Google Scholar]
- Grimminger H., Feldner J. Threonine deaminase from a mutant requiring isoleucine or pyridoxine: inability to form a tetrameric state. J Bacteriol. 1974 May;118(2):753–755. doi: 10.1128/jb.118.2.753-755.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger H., Lingens F. Alternate requirement for pyridoxine or isoleucine in mutants of Escherichia coli. FEBS Lett. 1969 Nov 12;5(3):225–226. doi: 10.1016/0014-5793(69)80338-8. [DOI] [PubMed] [Google Scholar]
- Grimminger H., Rahimi-Laridjani I., Koerner K., Lingens F. Purification of threonine deaminase from Escherichia coli. FEBS Lett. 1973 Sep 15;35(2):273–275. doi: 10.1016/0014-5793(73)80302-3. [DOI] [PubMed] [Google Scholar]
- Guirard B. M., Ames B. N., Snell E. E. Salmonella typhimurium mutants with alternate requirements for vitamin B 6 or isoleucine. J Bacteriol. 1971 Oct;108(1):359–363. doi: 10.1128/jb.108.1.359-363.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding W. M., Tubbs J. A., McDaniel D. Similar effects by valine and isoleucine on threonine deaminase. Can J Biochem. 1970 Jul;48(7):812–815. doi: 10.1139/o70-126. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Snell E. E. Crystalline L-histidinol phosphate aminotransferase from Salmonella typhimurium. Purification and subunit structure. J Biol Chem. 1973 Mar 25;248(6):1906–1911. [PubMed] [Google Scholar]
- Johnson G. S., Deal W. C., Jr Inactivation of tetrameric rabbit muscle pyruvate kinase by specific binding of 2 to 4 moles of pyridoxal 5'-phosphate. J Biol Chem. 1970 Jan 25;245(2):238–245. [PubMed] [Google Scholar]
- Koerner K., Rahimi-Laridjani I., Grimminger H. Purification of biosynthetic threonine deaminase from Escherichia coli. Biochim Biophys Acta. 1975 Jul 27;397(1):220–230. doi: 10.1016/0005-2744(75)90195-3. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Stallcup W. B., Koshland D. E., Jr Half-of-the-sites reactivity and the conformational states of cytidine triphosphate synthetase. Biochemistry. 1971 Aug 31;10(18):3371–3378. doi: 10.1021/bi00794a009. [DOI] [PubMed] [Google Scholar]
- MORRIS J. G., HUGHES D. T., MULDER C. Observations on the assay of vitamin B6 with Saccharomyces carlsbergensis 4228. J Gen Microbiol. 1959 Jun;20(3):566–575. doi: 10.1099/00221287-20-3-566. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Rahimi-Laridjani I., Grimminger H., Lingens F. Affinity chromatography of biosynthetic threonine deaminase of Escherichia coli. FEBS Lett. 1973 Mar 1;30(2):185–187. doi: 10.1016/0014-5793(73)80648-9. [DOI] [PubMed] [Google Scholar]
- Schnackerz K. D., Noltmann E. A. Pyridoxal 5'-phosphate as a site-specific protein reagent for a catalytically critical lysine residue in rabbit muscle phosphoglucose isomerase. Biochemistry. 1971 Dec 21;10(26):4837–4843. doi: 10.1021/bi00802a002. [DOI] [PubMed] [Google Scholar]
- Shizuta Y., Nakazawa A., Tokushige M., Hayaishi O. Studies on the interaction between regulatory enzymes and effectors. 3. Crystallization and characterization of adenosine 5'-monophosphate-dependent threonine deaminase from Escherichia coli. J Biol Chem. 1969 Apr 10;244(7):1883–1889. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E. Evidence for a negative-feedback mechanism in the biosynthesis of isoleucine. Science. 1956 May 11;123(3202):848–848. doi: 10.1126/science.123.3202.848. [DOI] [PubMed] [Google Scholar]
- WADA H., SNELL E. E. The enzymatic oxidation of pyridoxine and pyridoxamine phosphates. J Biol Chem. 1961 Jul;236:2089–2095. [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. Serine and threonine desaminaes of Escherichia coli; activators for a cell-free enzyme. J Biol Chem. 1949 Nov;181(1):171–182. [PubMed] [Google Scholar]
- Yanofsky C., Ito J., Horn V. Amino acid replacements and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:151–162. doi: 10.1101/sqb.1966.031.01.023. [DOI] [PubMed] [Google Scholar]


