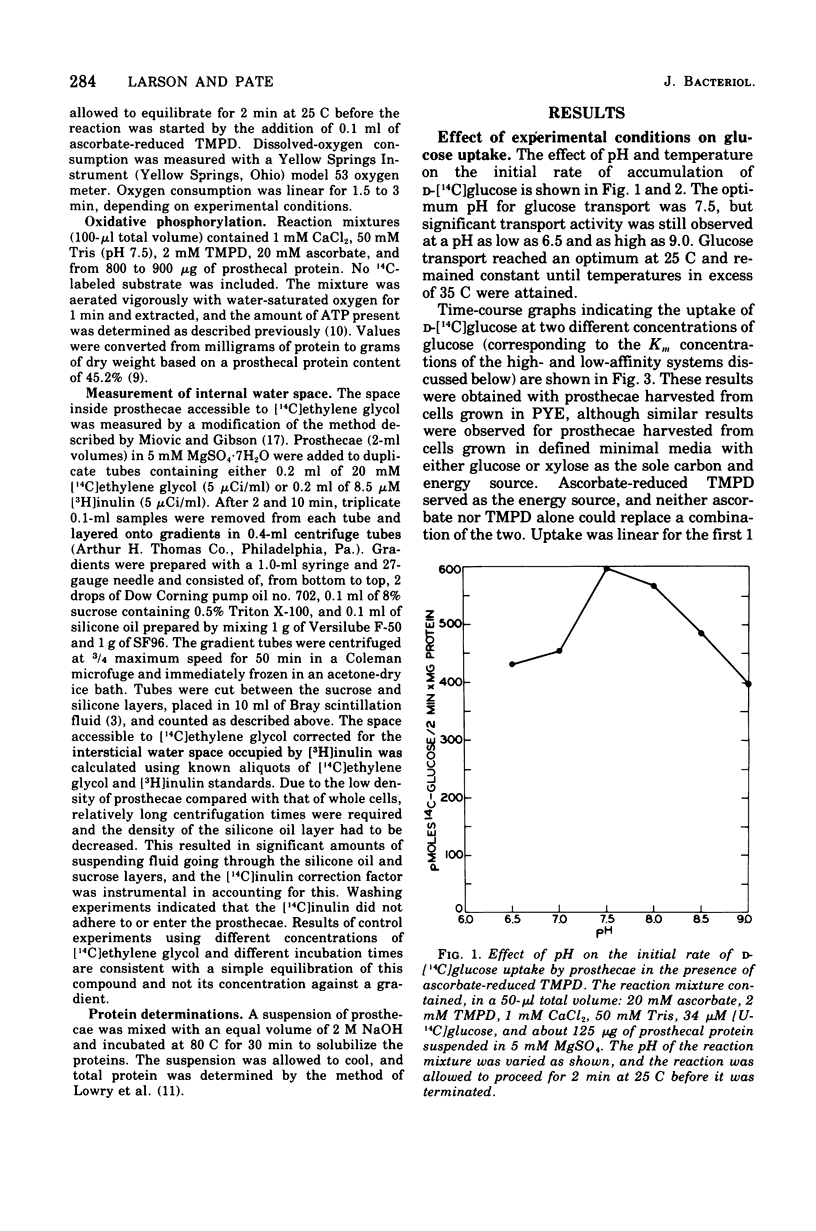
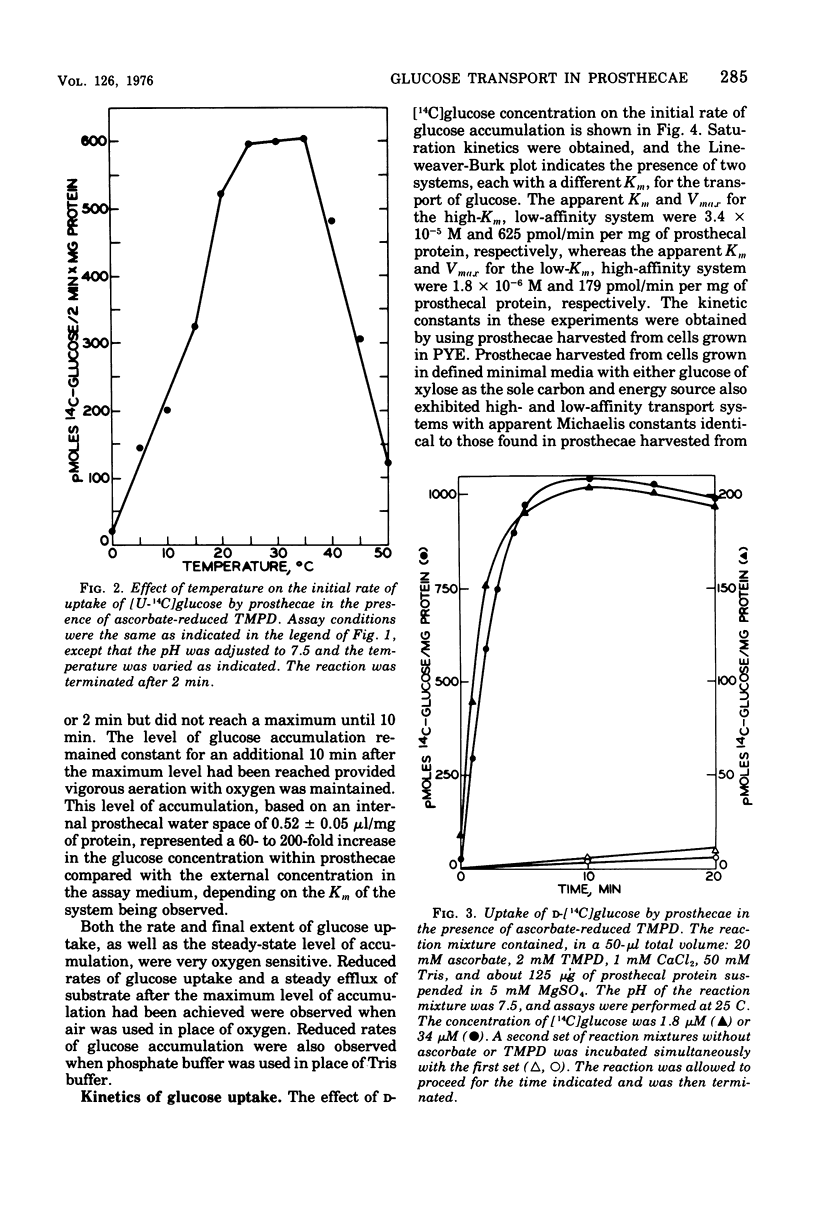
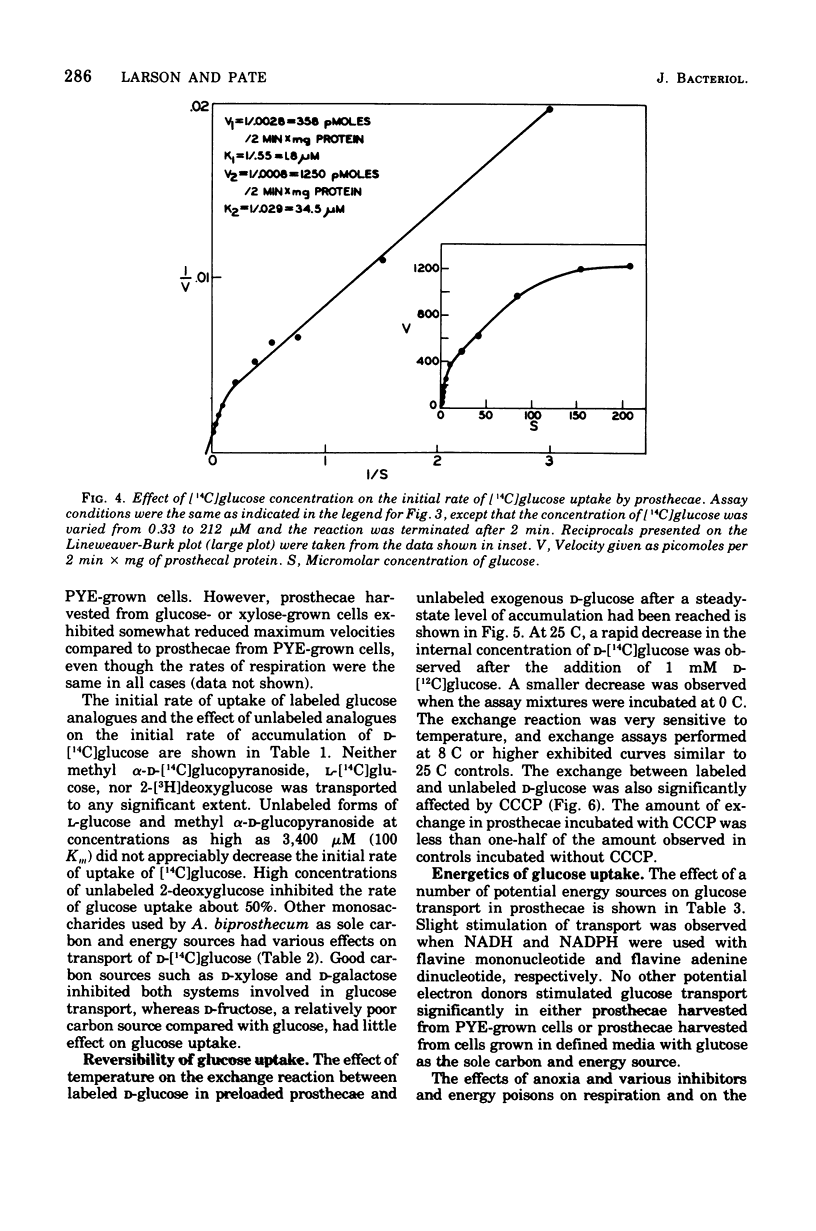
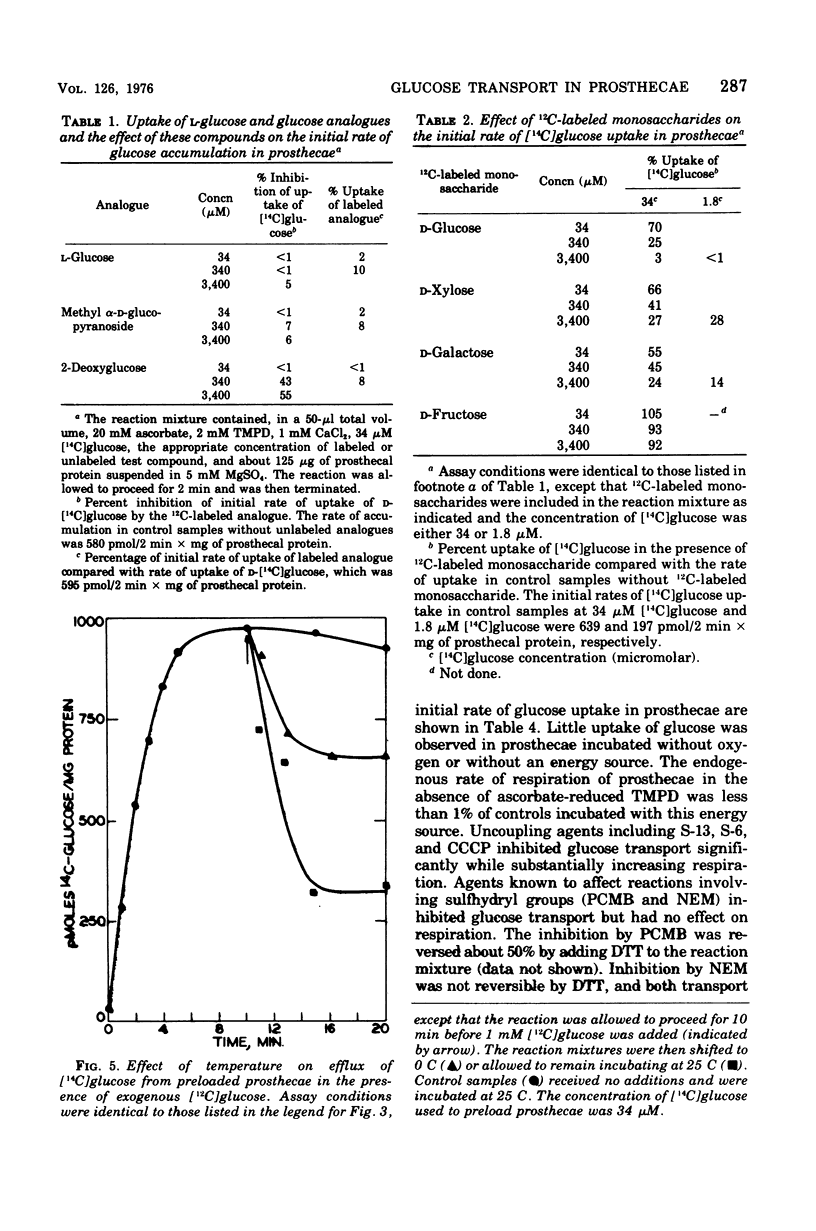
Abstract
Active transport of glucose in prosthecae isolated from cells of Asticcacaulis biprosthecum was stimulated by the non-physiological electron donor N, N, N', N'-tetramethyl-p-phenylenediamine dihydrochloride. Glucose uptake was mediated by two transport systems; the apparent Km of the high-affinity system was 1.8 muM and that of the low-affinity system was 34 muM. Free glucose accumulated within prosthecae at a concentration 60 to 200 times above that present externally, depending on the Km of the system being observed. The glucose transport system in prosthecae was stereospecific for D-glucose, and neither methyl alpha-D-glucopyranoside nor 2-deoxyglucose was transported. Uptake of glucose was inhibited by N-ethylmaleimide (NEM) and p-chloromercuribenzoate (PCMB), and the inhibition by PCMB but not by NEM was reversed by dithiothreitol. Glucose uptake was also inhibited by the uncoupling agents 5-chloro-3-t-butyl-2'-nitrosalicylanilide (S-13), 5-chloro-3-(p-chlorophenyl)-4'-chlorosalicylanilide (S-6), and carbonyl-cyanide m-chlorophenylhydrazone (CCCP) and by the respiratory inhibitor KCN. Efflux of glucose from preloaded prosthecae was induced by PCMB and KCN, but not by S-13 or CCCP. Glucose uptake was not affected by arsenate or an inhibitor of membrane-bound adenosine triphosphatases, N, N'-dicyclohexylcarbodiimide. The lack of inhibition by these two compounds, combined with the extremely low levels of adenosine 5'-triphosphate present in prosthecae, indicates that adenosine 5'-triphosphate is not involved in the transport of glucose by prosthecae.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr Respiration-coupled glucose transport in membrane vesicles from Azotobacter vinelandii. Arch Biochem Biophys. 1972 Oct;152(2):795–799. doi: 10.1016/0003-9861(72)90275-5. [DOI] [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Eagon R. G. Transport of glucose, gluconate, and methyl alpha-D-glucoside by Pseudomonas aeruginosa. J Bacteriol. 1974 Mar;117(3):1261–1269. doi: 10.1128/jb.117.3.1261-1269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORECKER B. L., THOMAS J., MONOD J. Galactose transport in Escherichia coli. I. General properties as studied in a galactokinaseless mutant. J Biol Chem. 1960 Jun;235:1580–1585. [PubMed] [Google Scholar]
- Holms W. H., Hamilton I. D., Robertson A. G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Arch Mikrobiol. 1972;83(2):95–109. doi: 10.1007/BF00425016. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. R., Segel I. H. Effect of weak acids on amino acid transport by Penicillium chrysogenum: evidence for a proton or charge gradient as the driving force. J Bacteriol. 1973 Mar;113(3):1184–1192. doi: 10.1128/jb.113.3.1184-1192.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan T. L., Porter J. S., Pate J. L. Isolation and characterization of prosthecae of Asticcacaulis biprosthecum. Arch Mikrobiol. 1974 Mar 1;96(1):1–16. doi: 10.1007/BF00590158. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Milner L. S. Relationship of a membrane-bound D-(-)-lactic dehydrogenase to amino acid transport in isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1008–1015. doi: 10.1073/pnas.66.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Reeves J. P., Short S. A., Lombardi F. J. Mechanisms of active transport in isolated bacterial membrane vesicles. 18. The mechanism of action of carbonylcyanide m-chlorophenylhydrazone. Arch Biochem Biophys. 1974 Jan;160(1):215–222. doi: 10.1016/s0003-9861(74)80028-7. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kerwar G. K., Gordon A. S., Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. IV. Galactose transport by isolated membrane vesicles from Escherichia coli. J Biol Chem. 1972 Jan 10;247(1):291–297. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miović M. L., Gibson J. Nucleotide pools and adenylate energy charge in balanced and unbalanced growth of Chromatium. J Bacteriol. 1973 Apr;114(1):86–95. doi: 10.1128/jb.114.1.86-95.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of two unusual stalked bacteria. J Cell Biol. 1965 Oct;27(1):133–150. doi: 10.1083/jcb.27.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Porter J. S., Jordan T. L. Asticcacaulis biprosthecum sp.nov. Life cycle, morphology and cultural characteristics. Antonie Van Leeuwenhoek. 1973 Nov;39(4):569–583. doi: 10.1007/BF02578901. [DOI] [PubMed] [Google Scholar]
- Porter J. S., Pate J. L. Prosthecae of Asticcacaulis biprosthecum: system for the study of membrane transport. J Bacteriol. 1975 Jun;122(3):976–986. doi: 10.1128/jb.122.3.976-986.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. P., Hong J. S., Kaback H. R. Reconstitution of D-lactate-dependent transport in membrane vesicles from a D-lactate dehydrogenase mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1917–1921. doi: 10.1073/pnas.70.7.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Robertson D. C. Glucose transport in Brucella abortus. J Bacteriol. 1974 Apr;118(1):250–258. doi: 10.1128/jb.118.1.250-258.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. Further studies on amino acid transport in Staphylococcus aureus membrane vesicles. J Biol Chem. 1974 Jul 10;249(13):4275–4281. [PubMed] [Google Scholar]
- Short S. A., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. Further studies on amino acid transport in Staphylococcus aureus membrane vesicles. J Biol Chem. 1974 Jul 10;249(13):4275–4281. [PubMed] [Google Scholar]
- Sobel M. E., Krulwich T. A. Metabolism of D-fructose by Arthrobacter pyridinolis. J Bacteriol. 1973 Feb;113(2):907–913. doi: 10.1128/jb.113.2.907-913.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F. The stoichiometry and site specificity of the uncoupling of mitochondrial oxidative phosphorylation by salicylanilide derivatives. Biochemistry. 1969 Jun;8(6):2475–2481. doi: 10.1021/bi00834a033. [DOI] [PubMed] [Google Scholar]


