Abstract
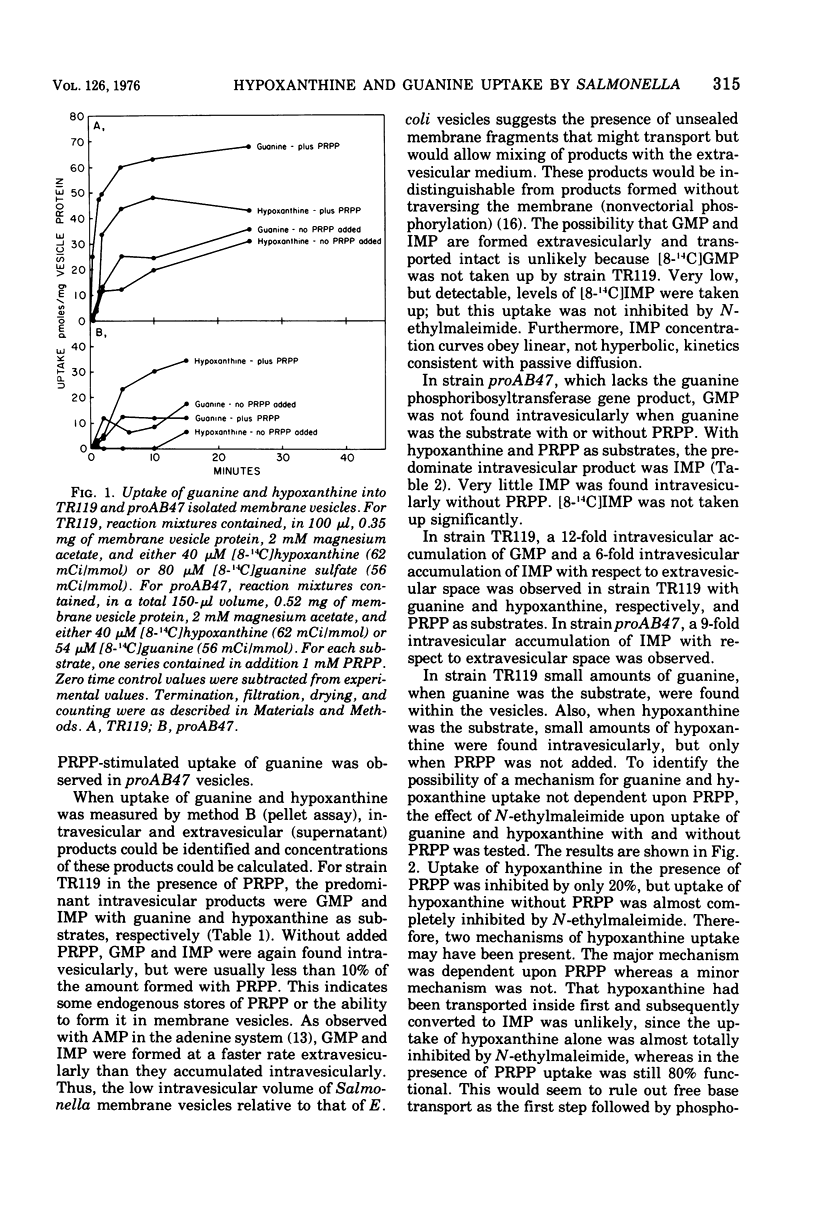
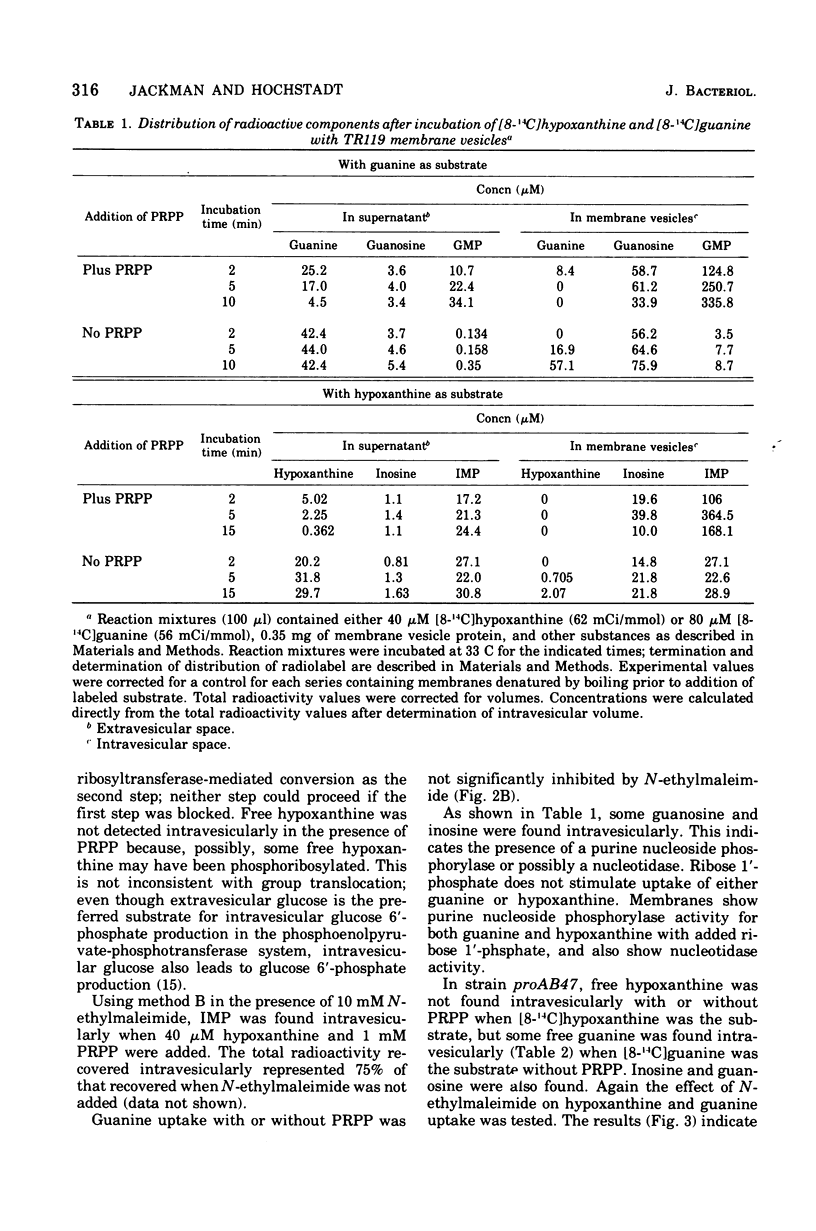
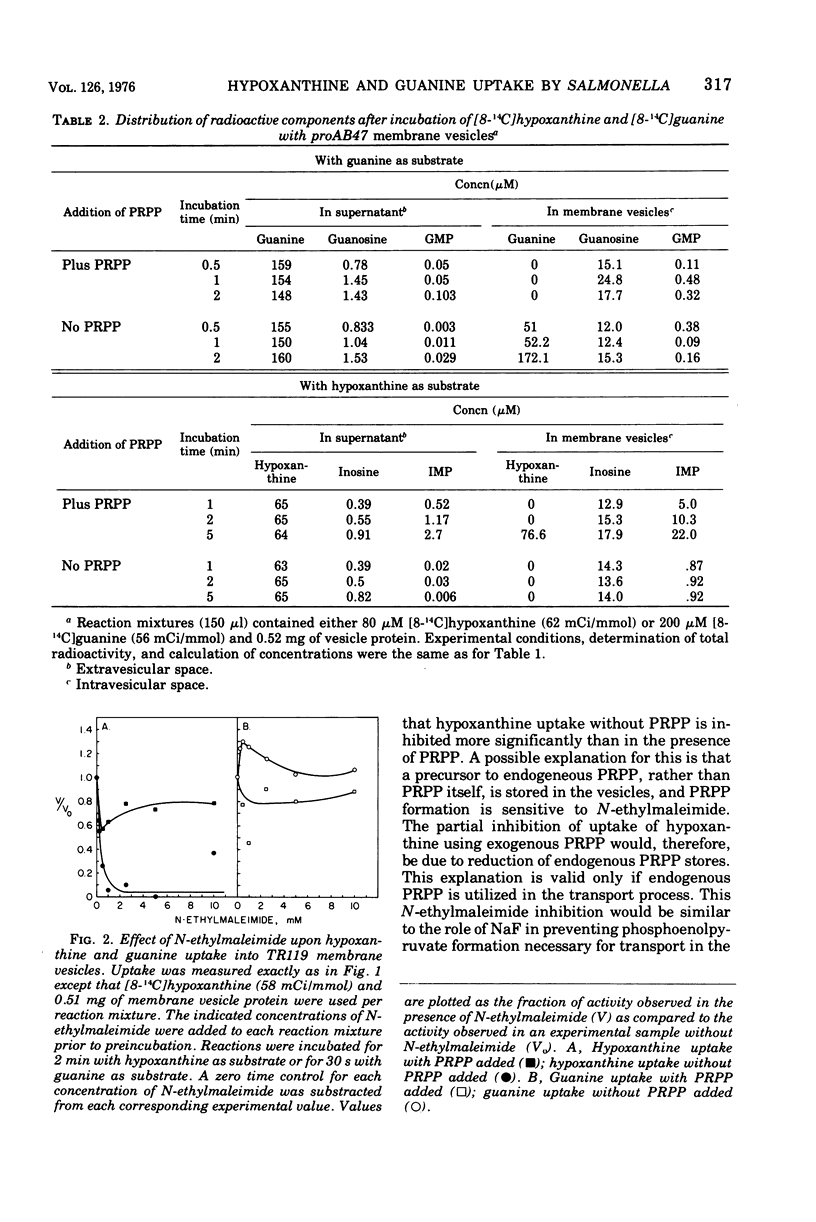
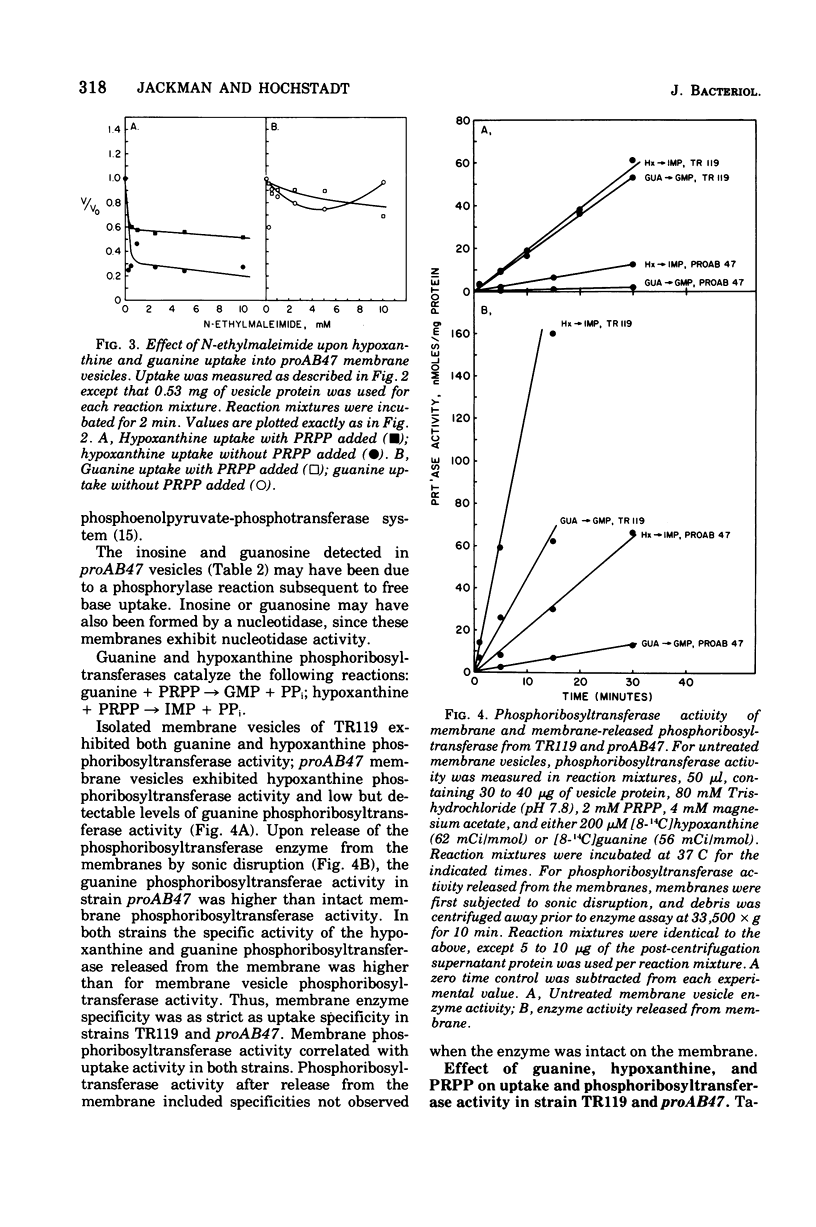
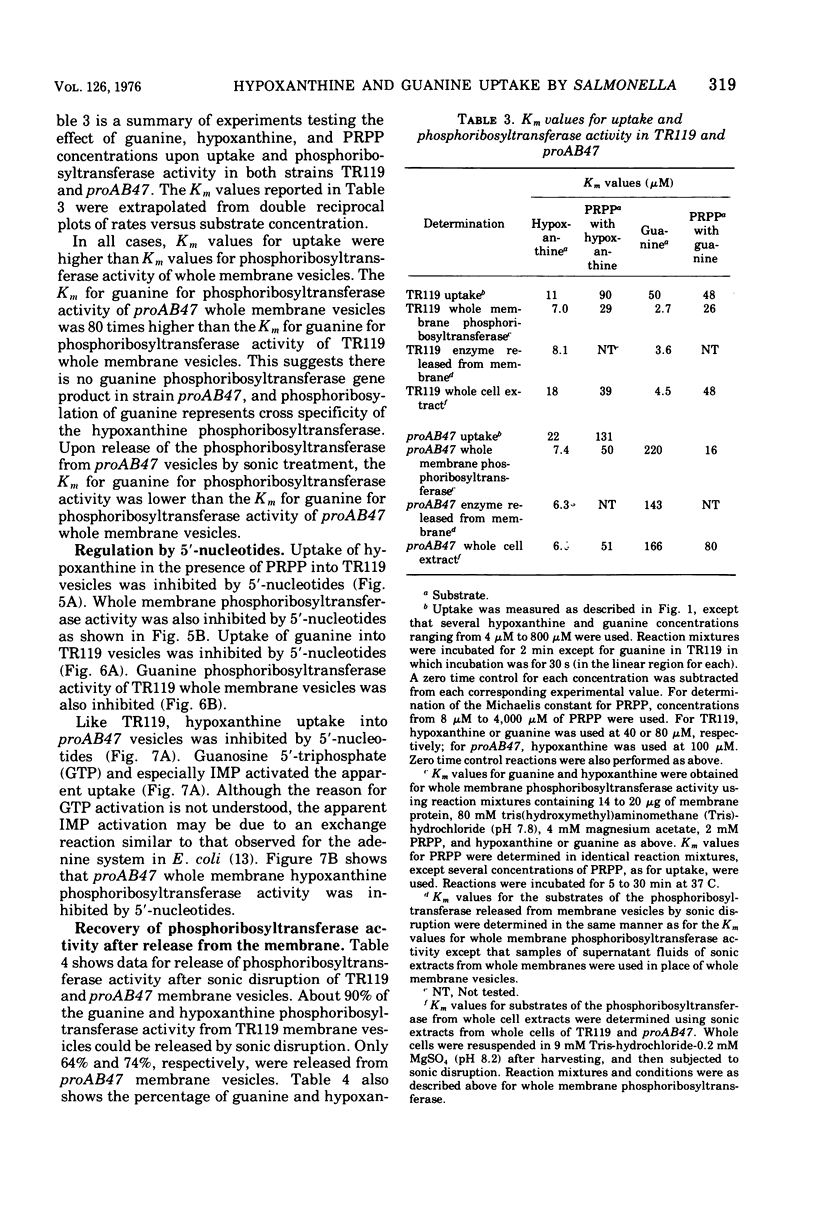
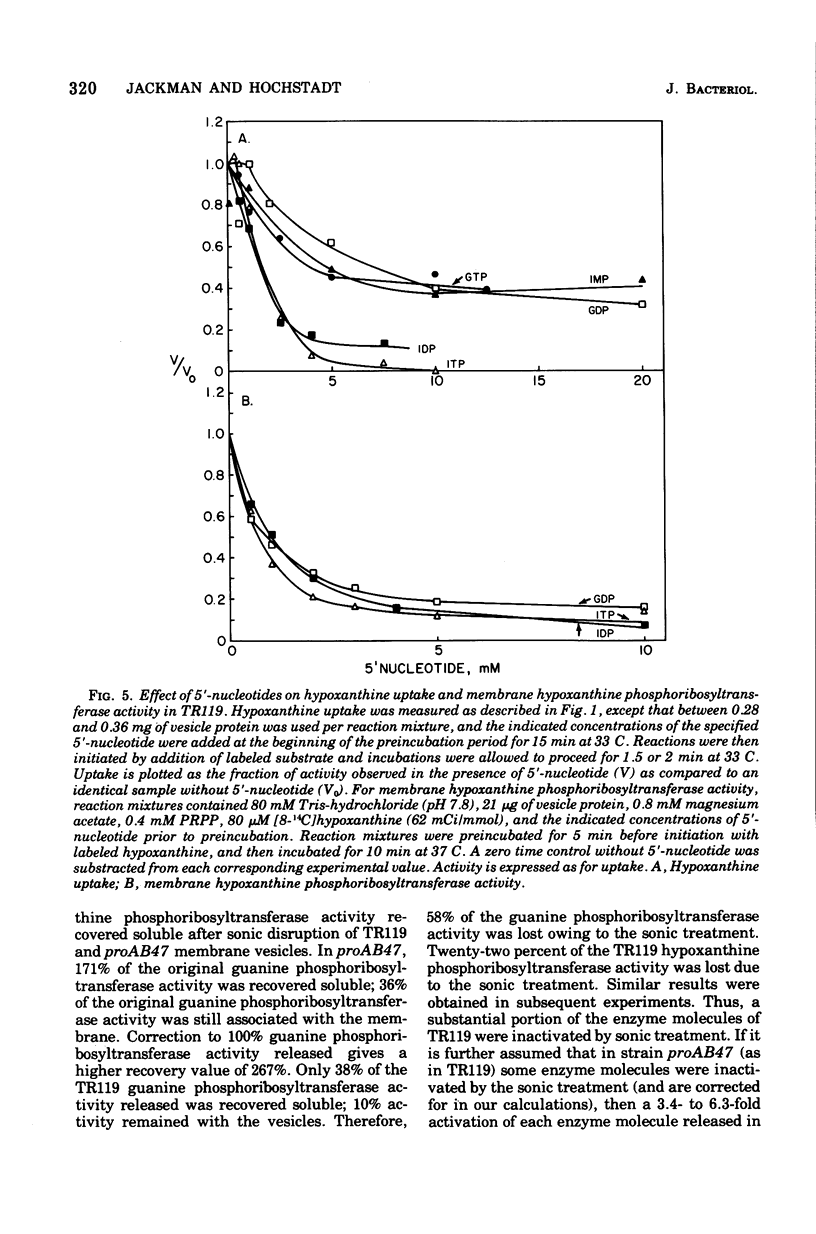
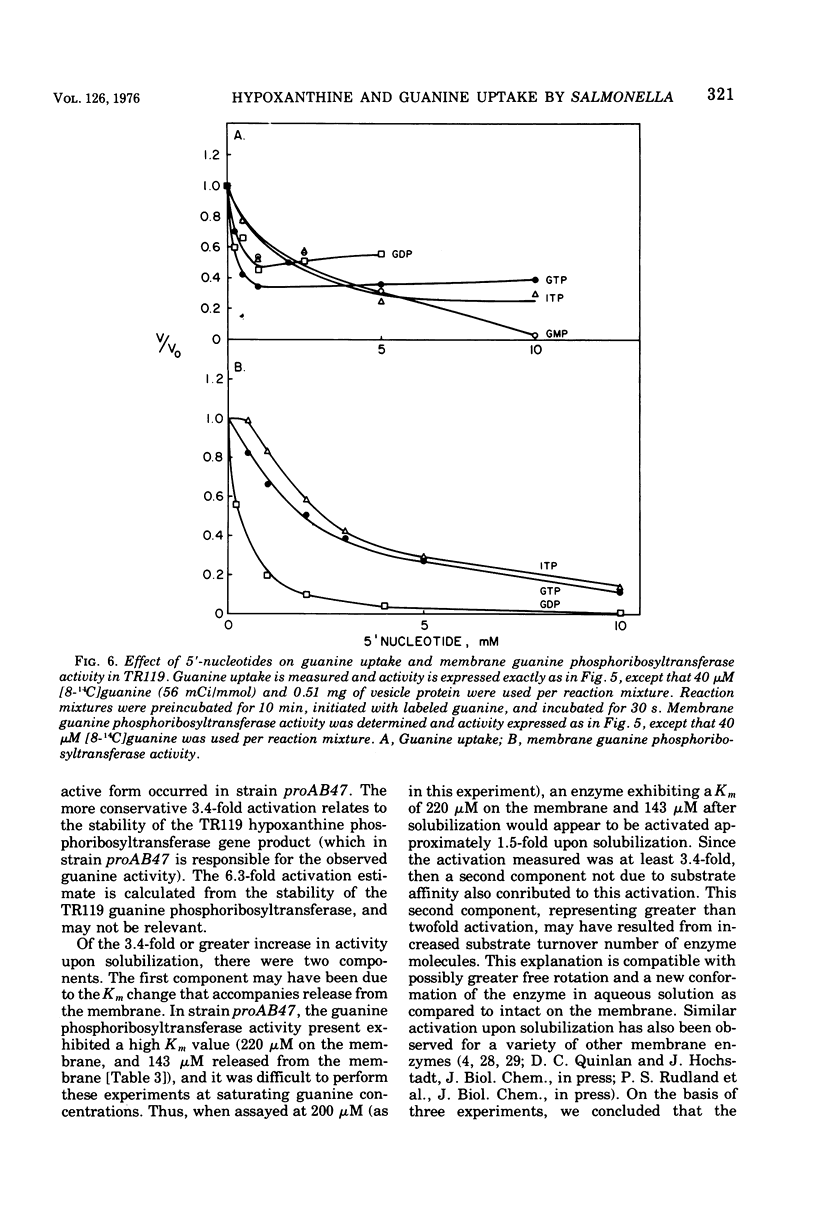
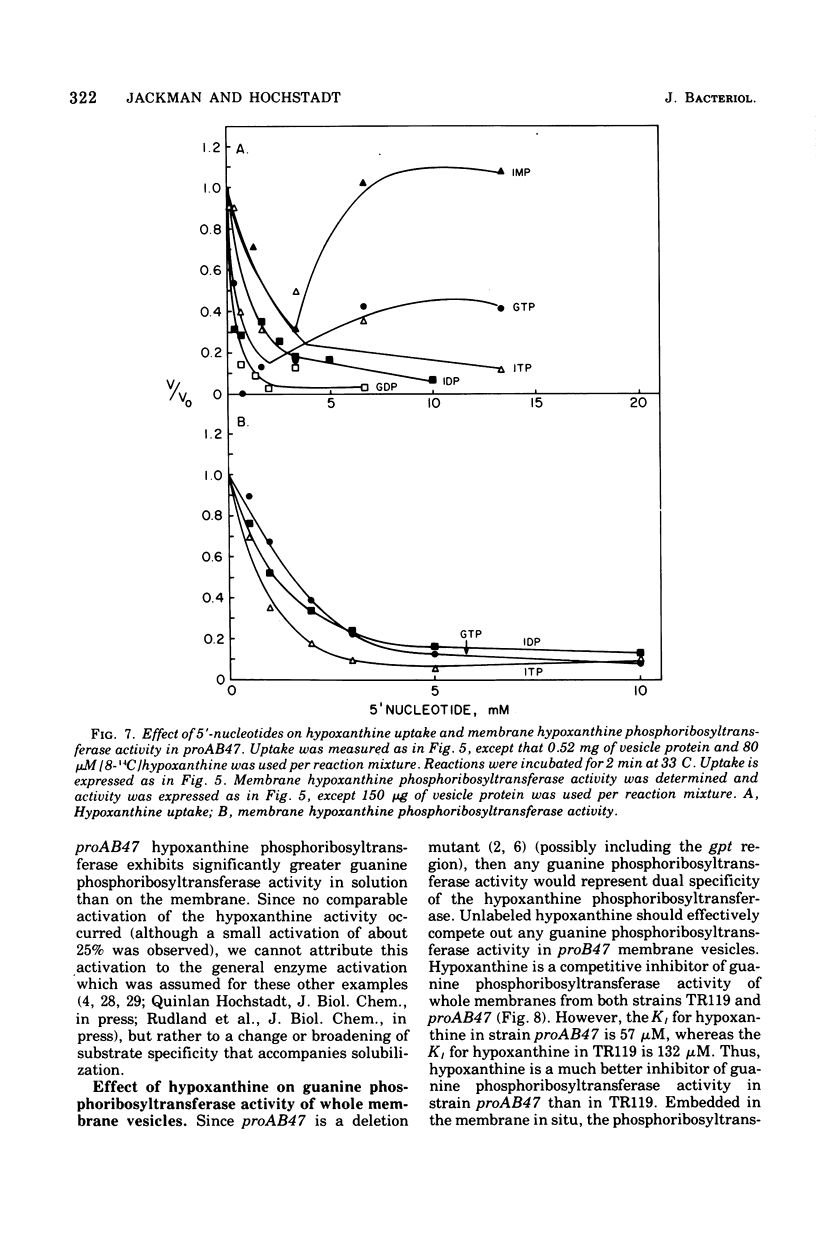
Uptake of hypoxanthine and guanine into isolated membrane vesicles of Salmonella typhimurium TR119 was stimulated by 5'-phosphoribosyl-1'-pyrophosphate (PRPP). For strain proAB47, a mutant that lacks guanine phosphoribosyltransferase, PRPP stimulated uptake of hypoxanthine into membrane vesicles. No PRPP-stimulated uptake of guanine was observed. For strain TR119, guanosine 5'-monophosphate and inosine 5'-monophosphate accumulated intravesicularly when guanine and hypoxanthine, respectively, were used with PRPP as transport substrates. For strain proAB47, IMP accumulated intravesicularly with hypoxanthine and PRPP as transport substrates. For strain TR119, hypoxanthine also accumulated when PRPP was absent. This free hypoxanthine uptake was completely inhibited by N-ethylmaleimide, but the PRPP-stimulated uptake of hypoxanthine was inhibited only 20% by N-ethylmaleimide. Hypoxanthine and guanine phosphoribosyltransferase activity paralleled uptake activity in both strains. But, when proAB47 vesicles were sonically treated to release the enzymes, a three- to sixfold activation of phosphoribosyltransferase molecules occurred. Since proAB47 vessicles lack the guanine phsophoribosyltransferase gene product and since hypoxanthine effectively competes out the phosphoribosylation of guanine by proAB47 vesicles, it was postulated that the hypoxanthine phosphoribosyltransferase gains specificity for both guanine and hypoxanthine when released from the membrane. A group translocation as the major mechanism for the uptake of guanine and hypoxanthine was proposed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr, Kaback H. R. Beta-galactoside transport in bacterial membrane preparations: energy coupling via membrane-bounded D-lactic dehydrogenase. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1190–1198. doi: 10.1073/pnas.66.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson C. E., Gots J. S. Genetic modification of substrate specificity of hypoxanthine phosphoribosyltransferase in Salmonella typhimurium. J Bacteriol. 1975 Jan;121(1):77–82. doi: 10.1128/jb.121.1.77-82.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Stadtman E. R. A possible role of purine nucleotide pyrophosphorylases in the regulation of purine uptake by Bacillus subtilis. J Biol Chem. 1966 Jun 10;241(11):2679–2686. [PubMed] [Google Scholar]
- Brooks D., Mays L. L., Hatefi Y., Young F. E. Glucosylation of teichoic acid: solubilization and partial characterization of the uridine diphosphoglucose: polyglycerolteichoic acid glucosyl transferase from membranes of Bacillus subtilis. J Bacteriol. 1971 Jul;107(1):223–229. doi: 10.1128/jb.107.1.223-229.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Purine phosphoribosyltransferases of Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):1010–1013. doi: 10.1128/jb.112.2.1010-1013.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Shumas S. R. Genetic separation of hypoxanthine and guanine-xanthine phosphoribosyltransferase activities by deletion mutations in Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):910–916. doi: 10.1128/jb.112.2.910-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilden S., Rhee H. M., Hokin L. E. Sodium transport by phospholipid vesicles containing purified sodium and potassium ion-activated adenosine triphosphatase. J Biol Chem. 1974 Dec 10;249(23):7432–7440. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Cashel M. The regulation of purine utilization in bacteria. V. Inhibition of purine phosphoribosyltransferase activities and purine uptake in isolated membrane vesicles by guanosine tetraphosphate. J Biol Chem. 1972 Nov 10;247(21):7067–7072. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. I. Purification of adenine phosphoribosyltransferase from Escherichia coli K12 and control of activity by nucleotides. J Biol Chem. 1971 Sep 10;246(17):5294–5303. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. II. Adenine phosphoribosyltransferase in isolated membrane preparations and its role in transport of adenine across the membrane. J Biol Chem. 1971 Sep 10;246(17):5304–5311. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. III. The involvement of purine phosphoribosyltransferases in the uptake of adenine and other nucleic acid precursors by intact resting cells. J Biol Chem. 1971 Sep 10;246(17):5312–5320. [PubMed] [Google Scholar]
- Hochstadt-Ozer J. The regulation of purine utilization in bacteria. IV. Roles of membrane-localized and pericytoplasmic enzymes in the mechanism of purine nucleoside transport across isolated Escherichia coli membranes. J Biol Chem. 1972 Apr 25;247(8):2419–2426. [PubMed] [Google Scholar]
- Hochstadt J. The role of the membrane in the utilization of nucleic acid precursors. CRC Crit Rev Biochem. 1974 Mar;2(2):259–310. doi: 10.3109/10409237409105449. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Kostellow A. B. Glycine uptake in Escherichia coli. I. Glycine uptake by whole cells of Escherichia coli W+ and a D-serine-resistant. J Biol Chem. 1968 Apr 10;243(7):1384–1389. [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Glycine uptake in Escherichia coli. II. Glycine uptake, exchange, and metabolism by an isolated membrane preparation. J Biol Chem. 1968 Apr 10;243(7):1390–1400. [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- Kaback H. R. Transport. Annu Rev Biochem. 1970;39:561–598. doi: 10.1146/annurev.bi.39.070170.003021. [DOI] [PubMed] [Google Scholar]
- Kerwar G. K., Gordon A. S., Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. IV. Galactose transport by isolated membrane vesicles from Escherichia coli. J Biol Chem. 1972 Jan 10;247(1):291–297. [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Miller R. L. Guanine and xanthine phosphoribosyltransfer activities of Lactobacillus casei and Escherichia coli. Their relationship to hypoxanthine and adenine phosphoribosyltransfer activities. J Biol Chem. 1970 May 25;245(10):2605–2611. [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller R. L., Ramsey G. A., Krenitsky T. A., Elion G. B. Guanine phosphoribosyltransferase from Escherichia coli, specificity and properties. Biochemistry. 1972 Dec 5;11(25):4723–4731. doi: 10.1021/bi00775a014. [DOI] [PubMed] [Google Scholar]
- Milner L. S., Kaback H. R. The role of phosphatidylglycerol in the vectorial phosphorylation of sugar by isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Mar;65(3):683–690. doi: 10.1073/pnas.65.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A. Thymidine breakdown and thymine uptake in different mutants of Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):228–237. doi: 10.1016/0005-2787(67)90530-8. [DOI] [PubMed] [Google Scholar]
- Pollock J. J., Linder R., Salton M. R. Characterization of the membrane-bound succinic dehydrogenase of Micrococcus lysodeikticus. J Bacteriol. 1971 Jul;107(1):230–238. doi: 10.1128/jb.107.1.230-238.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Hays J. B., Nakazawa T., Roseman S. Sugar transport. VI. Phosphoryl transfer in the lactose phosphotransferase system of Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):957–965. [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Yagil E., Beacham I. R. Uptake of adenosine 5'-monophosphate by Escherichia coli. J Bacteriol. 1975 Feb;121(2):401–405. doi: 10.1128/jb.121.2.401-405.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN E. F., MAGASANIK B. UTILIZATION AND INTERCONVERSION OF PURINE BASES AND RIBONUCLEOSIDES BY SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jan;239:293–300. [PubMed] [Google Scholar]


