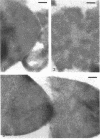
Abstract
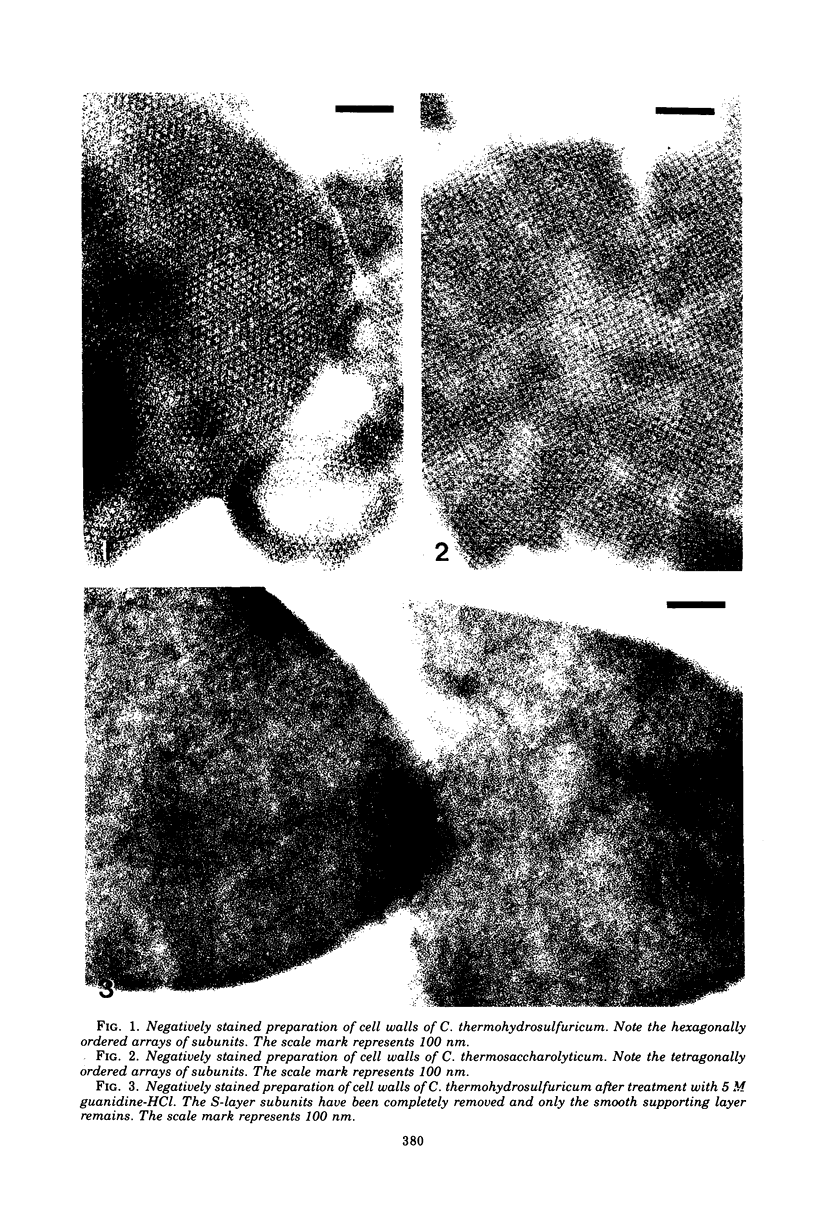
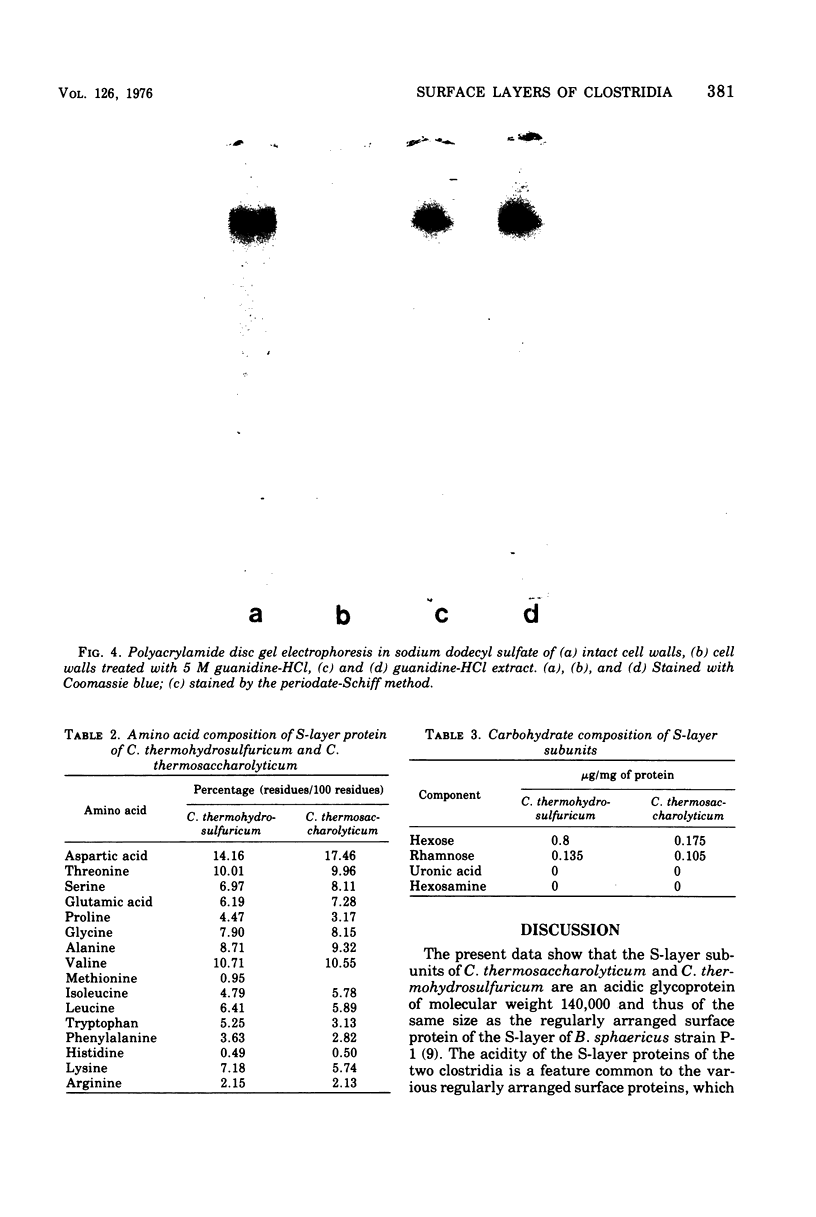
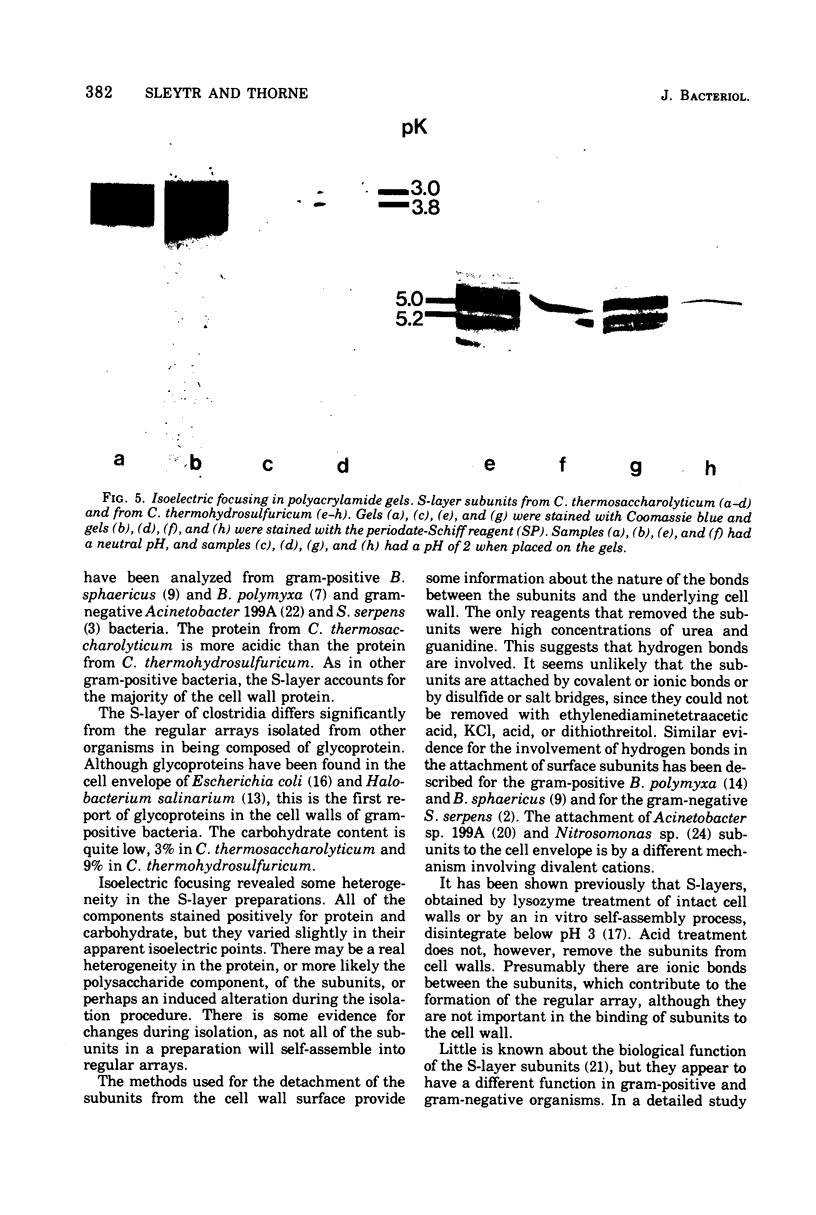
Clostridum thermosaccharolyticum and Clostridium thermohydrosulfuricum possess as outermost cell wall layer a tetragonal or hexagonal ordered array of macromolecules. The subunits of the surface layer can be detached from isolated cell walls with urea (8M) or guanidine-HCl (4 to 5 M). Triton X-100, dithiothreitol, ethylenediaminetetracetate, and KCl (3 M) had no visible effect on the regular arrays. Sodium dodecyl sulfate-polyacrylamide electrophroesis showed that, in both organisms, the surface layer is composed of glycoprotein of molecular weight 140,000. The glycoprotein from both microorganisms has a predominantly acidic amino acid composition and an acidic isoelectric point after isoelectric focusing on polyacrylamide gels. The glycocomponent is composed of glucose, galactose, mannose, and rhamnose.
Full text
PDF


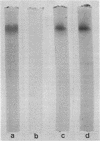
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Glaubert A. M., Sleytr U. B. Analysis of regular arrays of subunits on bacterial surfaces: evidence for a dynamic process of assembly. J Ultrastruct Res. 1975 Jan;50(1):103–116. doi: 10.1016/s0022-5320(75)90012-x. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. Fine structure and radiation resistance in Acinetobacter: a comparison of a range of strains. J Cell Sci. 1971 Jan;8(1):19–41. doi: 10.1242/jcs.8.1.19. [DOI] [PubMed] [Google Scholar]
- Hollaus F., Sleytr U. On the taxonomy and fine structure of some hyperthermophilic saccharolytic Clostridia. Arch Mikrobiol. 1972;86(2):129–146. doi: 10.1007/BF00413368. [DOI] [PubMed] [Google Scholar]
- Howard L., Tipper D. J. A polypeptide bacteriophage receptor: modified cell wall protein subunits in bacteriophage-resistant mutants of Bacillus sphaericus strain P-1. J Bacteriol. 1973 Mar;113(3):1491–1504. doi: 10.1128/jb.113.3.1491-1504.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L., Watson S. W. Protein and carbohydrate composition of the cell envelope of Halobacterium salinarium. J Bacteriol. 1974 Nov;120(2):945–954. doi: 10.1128/jb.120.2.945-954.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S., Weinbaum G. An envelope-specific glycoprotein from Escherichia coli B. Biochemistry. 1968 Aug;7(8):2819–2825. doi: 10.1021/bi00848a018. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Heterologous reattachment of regular arrays of glycoproteins on bacterial surfaces. Nature. 1975 Oct 2;257(5525):400–402. doi: 10.1038/257400a0. [DOI] [PubMed] [Google Scholar]
- Sleytr U., Adam H., Klaushofer H. Die Feinstruktur der Zellwandoberfläche von zwei thermophilen Clostridienarten, dargestellt mit Hilfe der Gefrierätztechnik. Mikroskopie. 1968 Aug;23(1):1–10. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Thornley M. J., Naisbitt P., Glauert A. M. The nature of the attachment of a regularly arranged surface protein to the outer membrane of an Acinetobacter sp. Biochim Biophys Acta. 1975 Apr 21;389(1):97–116. doi: 10.1016/0005-2736(75)90388-0. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M., Sleytr U. B. Structure and assembly of bacterial surface layers composed of regular arrays of subunits. Philos Trans R Soc Lond B Biol Sci. 1974 Jul 25;268(891):147–153. doi: 10.1098/rstb.1974.0022. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Thorne K. J., Glauert A. M. Detachment and chemical characterization of the regularly arranged subunits from the surface of an Acinetobacter. J Bacteriol. 1974 May;118(2):654–662. doi: 10.1128/jb.118.2.654-662.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Remsen C. C. Macromolecular subunits in the walls of marine nitrifying bacteria. Science. 1969 Feb 14;163(3868):685–686. doi: 10.1126/science.163.3868.685. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]