Abstract
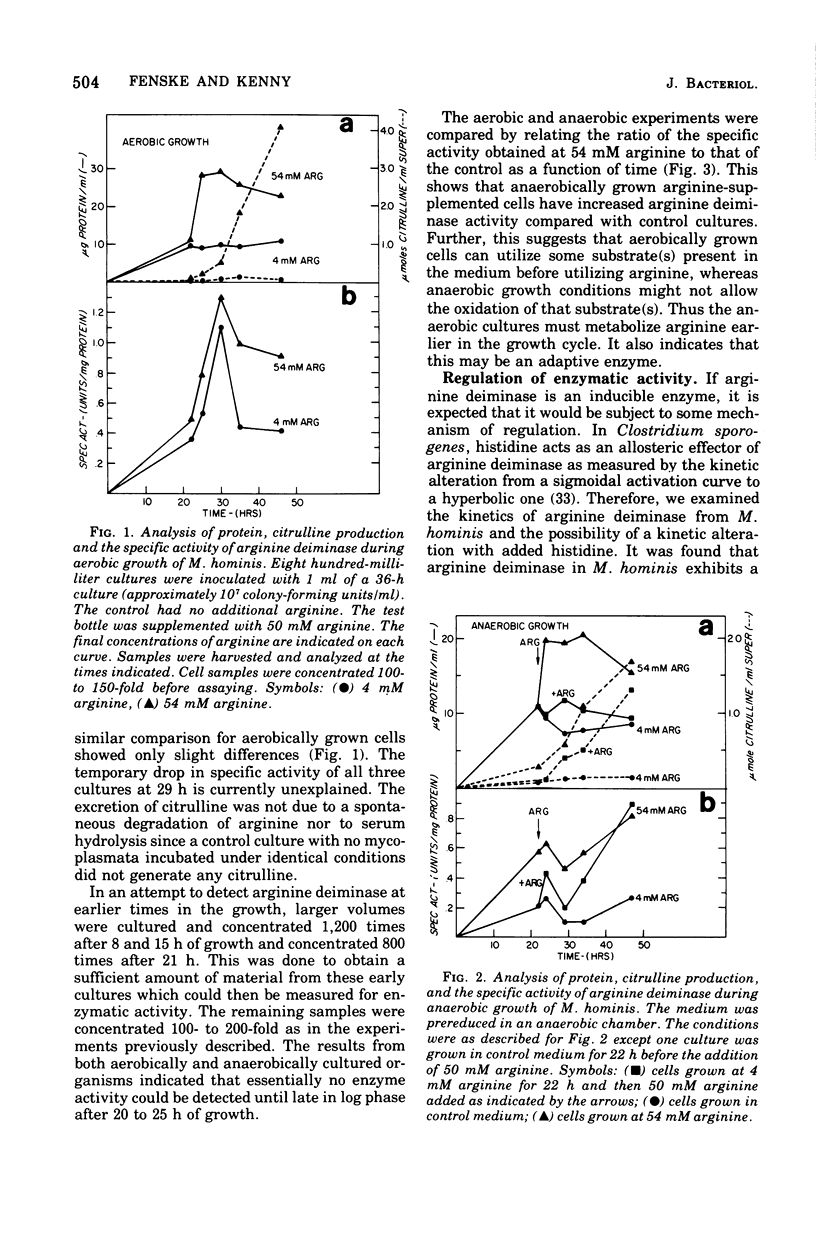
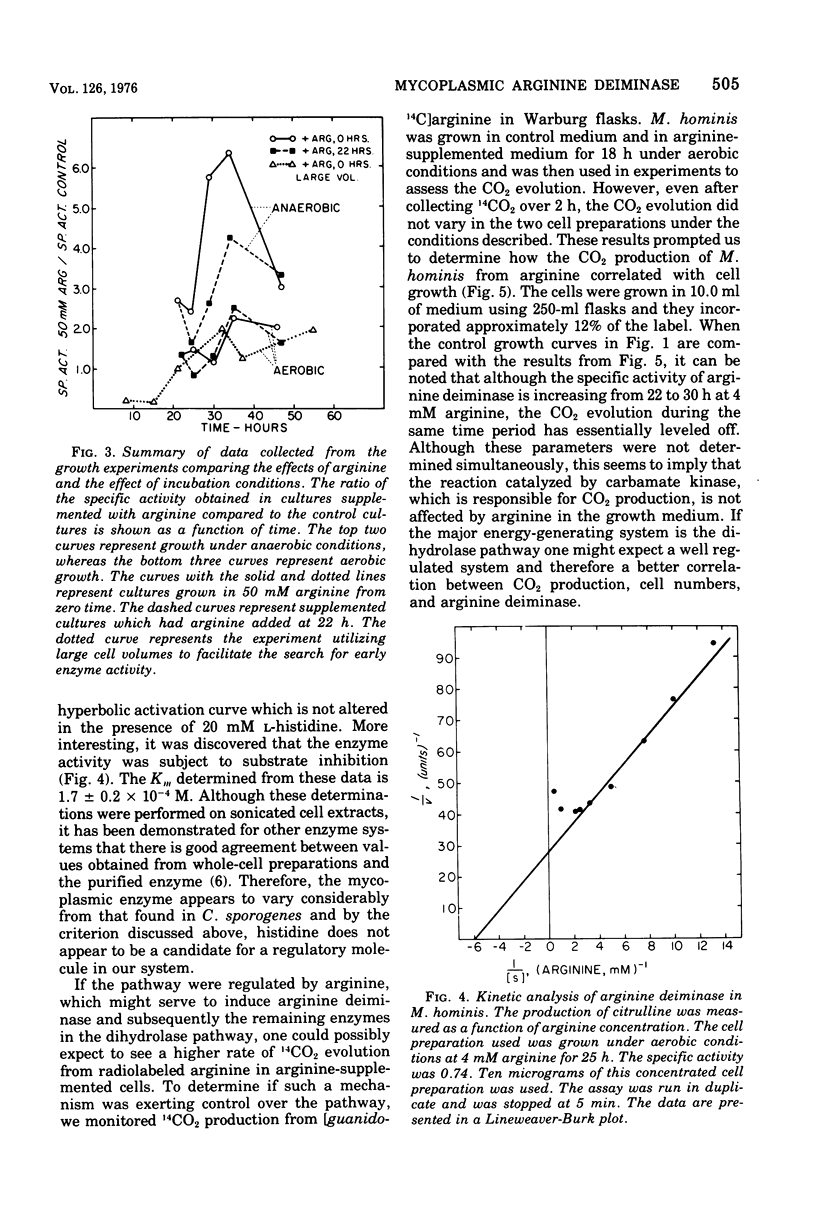
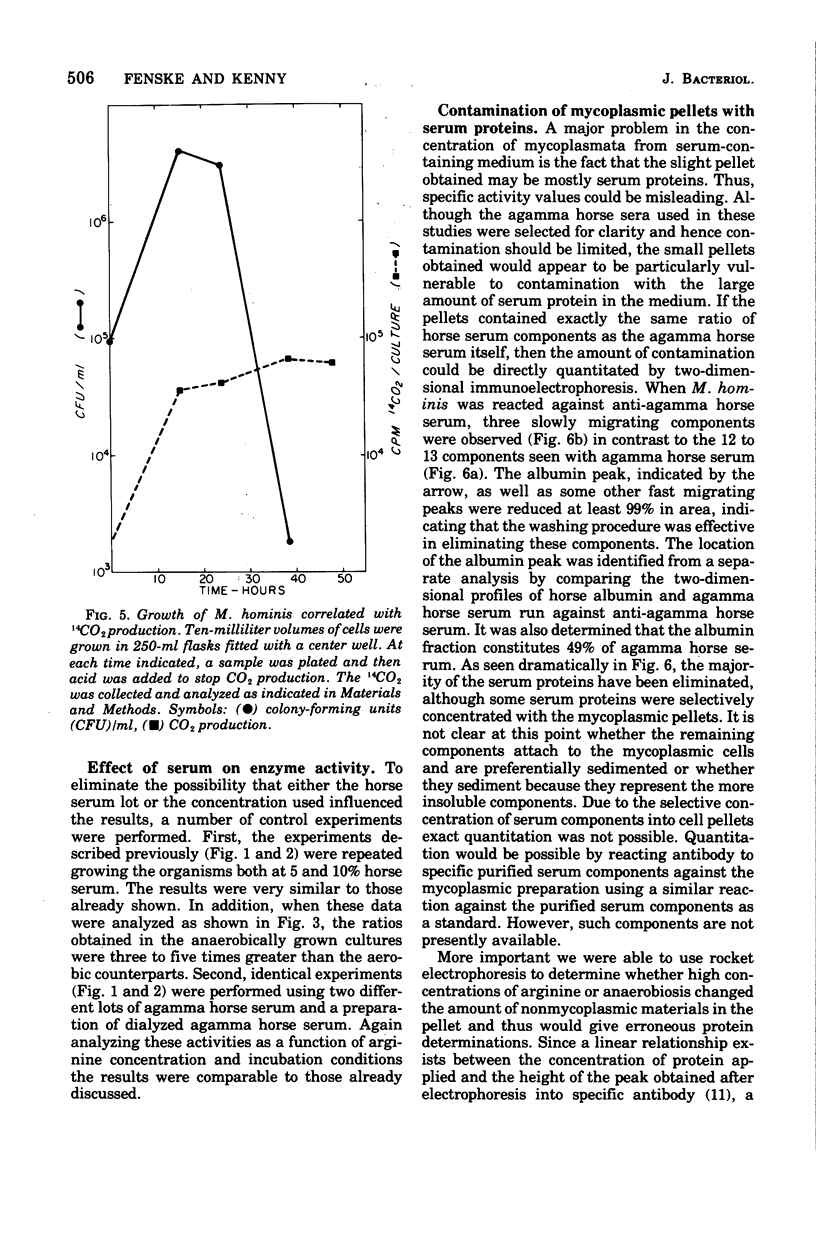
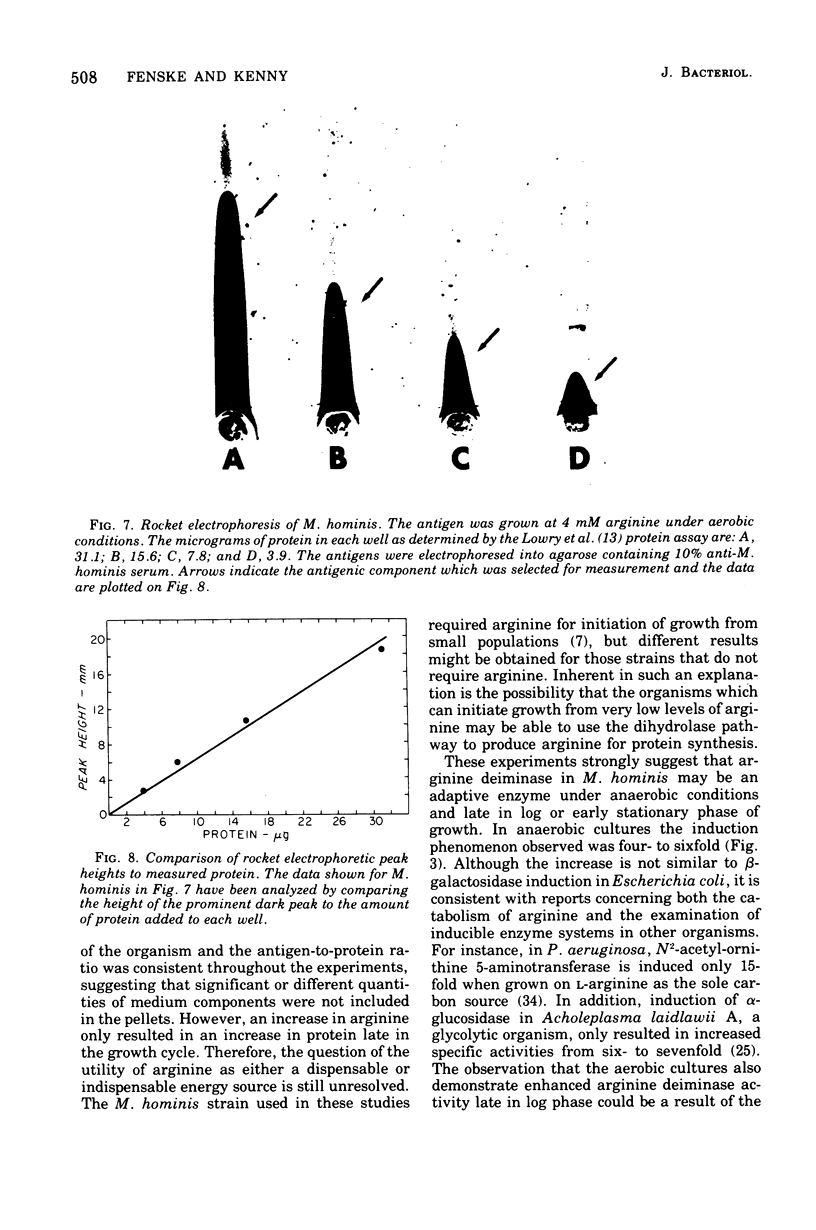
Arginine has been considered as the major energy source of nonglycolytic arginine-utilizing mycoplasmata. When three strains of Mycoplasma arginini, and one strain each of Mycoplasma arthritidis, Mycoplasma fermentans, Mycoplasma gallinarum, Mycoplasma gallisepticum and Mycoplasma hominis were grown in the medium with high arginine concentration (34 mM) compared with low arginine (4 mM), both the protein content of the organisms and the specific activity of arginine deiminase increased. M. fermentans, the one arginine-utilizing species included in the survey which is also glycolytic, showed an increase in protein content but no increase in specific activity of the enzyme. The glycolytic non-arginine-utilizing M. gallisepticum did not show an increase in either parameter. The Km for arginine deiminase from crude cell extracts was 1.66 X 10(-4)M. The enzyme demonstrated a hyperbolic activation curve subject to substrate inhibition and was not affected by the presence of L-histidine. When mycoplasmic protein and arginine deiminase were determined for M. hominis under aerobic and anaerobic conditions, aerobically grown cells exhibited no detectable enzymatic increases until late in log phase. Higher levels of arginine deiminase were observed earlier in the anaerobic growth cycle. The rate of 14CO2 evolution from [guanido-14C]arginine was not altered in arginine-supplemented cells compared with cells grown in low arginine. In addition, CO2 production did not parallel increased arginine deiminase activity. These observations argue that arginine is used only as an alternate energy source in these organisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajello F., Romano N. Effect of L-histidine on the survival of a T-strain of mycoplasma. Appl Microbiol. 1975 Feb;29(2):293–294. doi: 10.1128/am.29.2.293-294.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., Schimke R. T., Riggs D. B. Presence of the arginine dihydrolase pathway in Mycoplasma. J Bacteriol. 1966 Jan;91(1):189–192. doi: 10.1128/jb.91.1.189-192.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S. K., DeMoss R. D. Physiological comparison of L-serine dehydratase and tryptophanase from Bacillus alvei. J Bacteriol. 1970 Mar;101(3):813–820. doi: 10.1128/jb.101.3.813-820.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn R. G., Kenny G. E. Differences in arginine requirement for growth among arginine-utilizing Mycoplasma species. J Bacteriol. 1974 Feb;117(2):611–618. doi: 10.1128/jb.117.2.611-618.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNY G. E., POLLOCK M. E. Mammalian cell cultures contaminated with pleuropneumonia-like organisms. I. Effect of pleuropneumonia-like organisms on growth of established cell strains. J Infect Dis. 1963 Jan-Feb;112:7–16. doi: 10.1093/infdis/112.1.7. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Serological comparison of ten glycolytic Mycoplasma species. J Bacteriol. 1969 Jun;98(3):1044–1055. doi: 10.1128/jb.98.3.1044-1055.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ottow J. C. Arginine dihydrolase activity in species of the genus Bacillus revealed by thin-layer chromatography. J Gen Microbiol. 1974 Sep;84(1):209–213. doi: 10.1099/00221287-84-1-209. [DOI] [PubMed] [Google Scholar]
- Pollock M. E., Bonner S. V. Comparison of undefined medium and its dialyzable fraction for growth of Mycoplasma. J Bacteriol. 1969 Feb;97(2):522–525. doi: 10.1128/jb.97.2.522-525.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROCHE J., LACOMBE G. Sur l'argininedésiminase et sur la formation enzymatique de citrulline par les levures. Biochim Biophys Acta. 1952 Dec;9(6):687–692. doi: 10.1016/0006-3002(52)90230-8. [DOI] [PubMed] [Google Scholar]
- Razin S. Physiology of mycoplasmas. Adv Microb Physiol. 1973;10:1–80. doi: 10.1016/s0065-2911(08)60086-7. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., BARILE M. F. ARGININE METABOLISM IN PLEUROPNEUMONIA-LIKE ORGANISMS ISOLATED FROM MAMMALIAN CELL CULTURE. J Bacteriol. 1963 Aug;86:195–206. doi: 10.1128/jb.86.2.195-206.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERRIS J. C., PRESTON N. W., SHOESMITH J. G. The influence of oxygen and arginine on the motility of a strain of Pseudomonas sp. J Gen Microbiol. 1957 Feb;16(1):86–96. doi: 10.1099/00221287-16-1-86. [DOI] [PubMed] [Google Scholar]
- SHOESMITH J. H., SHERRIS J. C. Studies on the mechanism of arginine-activated motility in a Pseudomonas strain. J Gen Microbiol. 1960 Feb;22:10–24. doi: 10.1099/00221287-22-1-10. [DOI] [PubMed] [Google Scholar]
- SLADE H. D., SLAMP W. C. The formation of arginine dihydrolase by streptococci and some properties of the enzyme system. J Bacteriol. 1952 Oct;64(4):455–466. doi: 10.1128/jb.64.4.455-466.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F. Amino acid metabolism by pleuropneumonialike organisms. I. General catabolism. J Bacteriol. 1955 Nov;70(5):552–556. doi: 10.1128/jb.70.5.552-556.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F. Conversion of citrulline to ornithine by pleuropneumonialike organisms. J Bacteriol. 1957 Dec;74(6):801–806. doi: 10.1128/jb.74.6.801-806.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Berlin C. M., Sweeney E. W., Carroll W. R. The generation of energy by the arginine dihydrolase pathway in Mycoplasma hominis 07. J Biol Chem. 1966 May 25;241(10):2228–2236. [PubMed] [Google Scholar]
- Slater M. L., Folsome C. E. Induction of alpha-glucosidase in Mycoplasma laidlawii A. Nat New Biol. 1971 Jan 27;229(4):117–118. doi: 10.1038/newbio229117a0. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Thirkill C. E., Kenny G. E. Serological comparison of five arginine-utilizing Mycoplasma species by two-dimensional immunoelectrophoresis. Infect Immun. 1974 Sep;10(3):624–632. doi: 10.1128/iai.10.3.624-632.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDEMARK P. J., SMITH P. F. EVIDENCE FOR A TRICARBOXYLIC ACID CYCLE IN MYCOPLASMA HOMINIS. J Bacteriol. 1964 Dec;88:1602–1607. doi: 10.1128/jb.88.6.1602-1607.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDEMARK P. J., SMITH P. F. NATURE OF BUTYRATE OXIDATION BY MYCOPLASMA HOMINIS. J Bacteriol. 1965 Feb;89:373–377. doi: 10.1128/jb.89.2.373-377.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDEMARK P. J., SMITH P. F. RESPIRATORY PATHWAYS IN THE MYCOPLASMA. II. PATHWAY OF ELECTRON TRANSPORT DURING OXIDATION OF REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE BY MYCOPLASMA HOMINIS. J Bacteriol. 1964 Jul;88:122–129. doi: 10.1128/jb.88.1.122-129.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal V., Doke S. N., Harikumar P., Kumta U. S. Histidine-dependent activation of arginine deiminase in Clostridium sporogenes: kinetic evidence on in vivo allosteric interactions. FEBS Lett. 1975 Mar 1;51(1):246–248. doi: 10.1016/0014-5793(75)80897-0. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Leisinger T. Dual role for N-2-acetylornithine 5-aminotransferase from Pseudomonas aeruginosa in arginine biosynthesis and arginine catabolism. J Bacteriol. 1975 Jun;122(3):799–809. doi: 10.1128/jb.122.3.799-809.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Sato T., Tosa T., Chibata I. Continuous production of L-citrulline by immobilized Pseudomonas putida cells. Biotechnol Bioeng. 1974 Dec;16(12):1589–1599. doi: 10.1002/bit.260161203. [DOI] [PubMed] [Google Scholar]