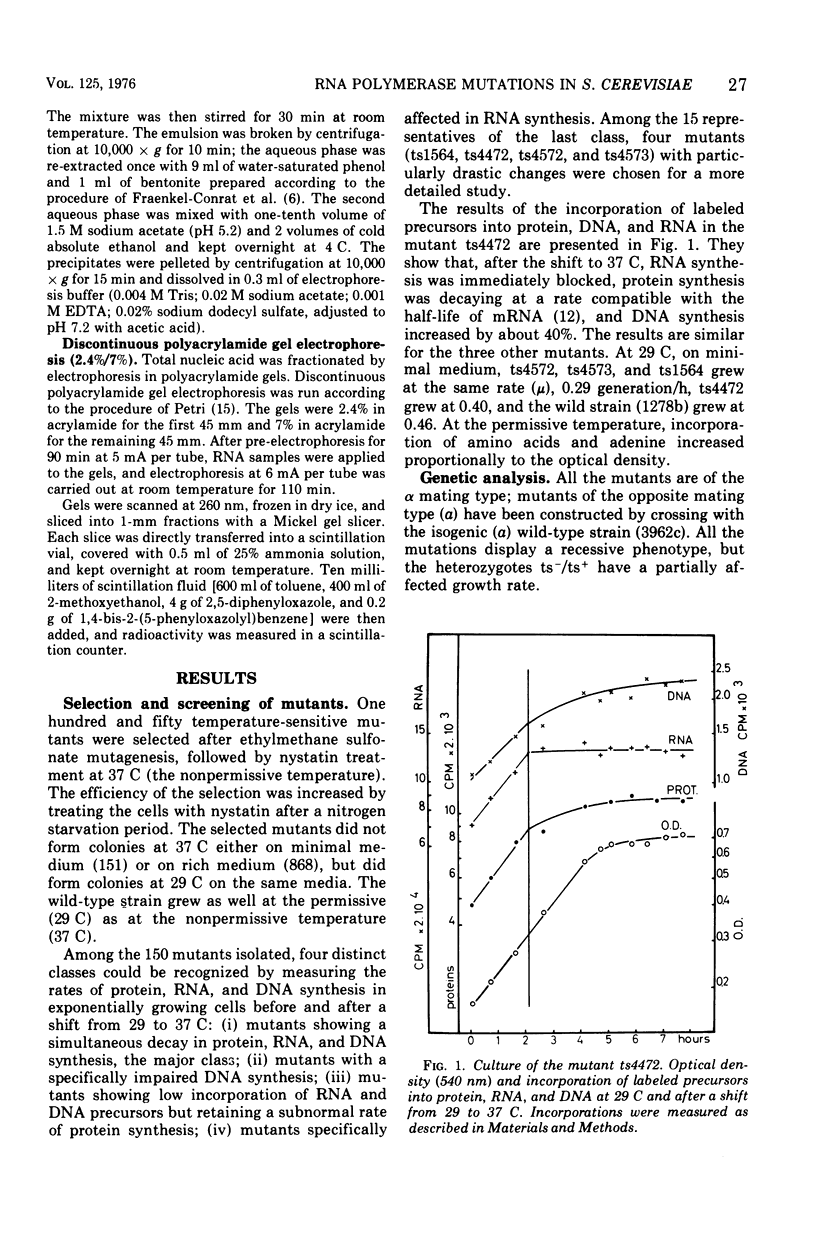
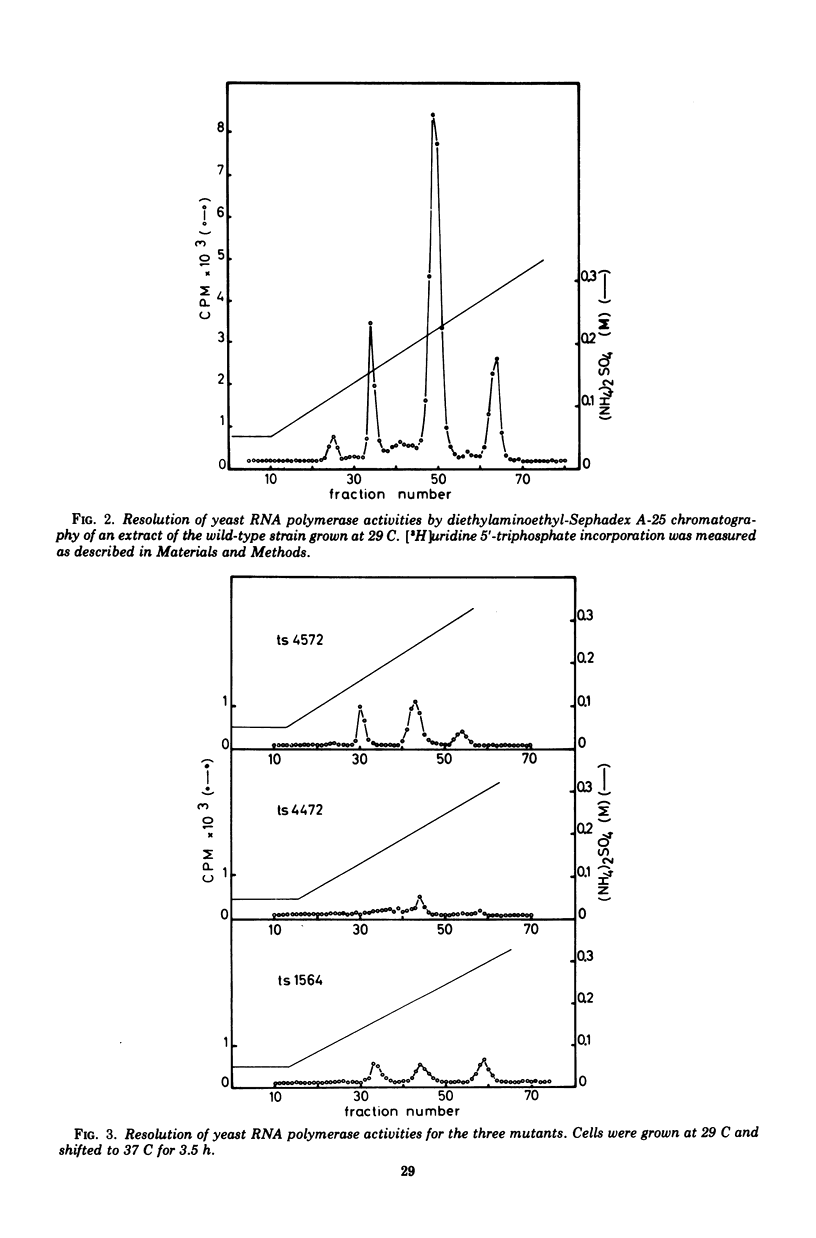
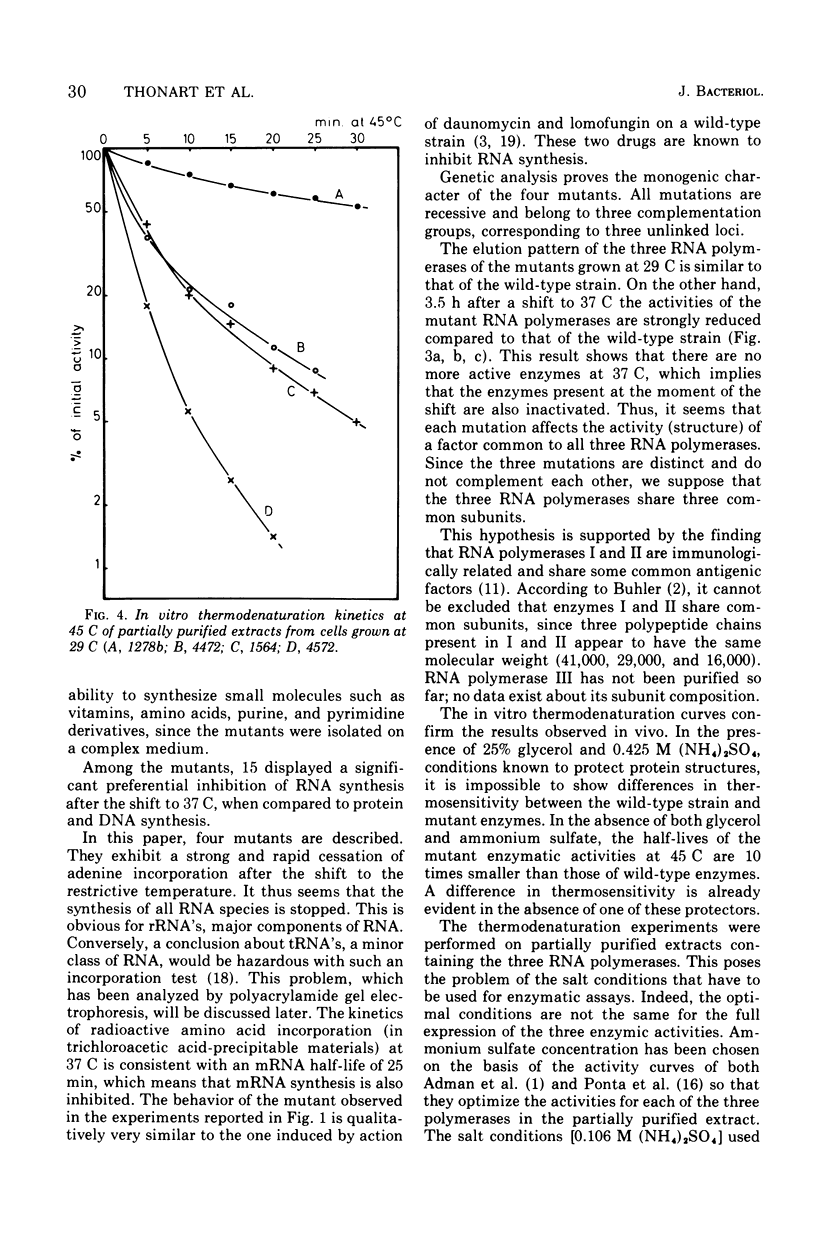
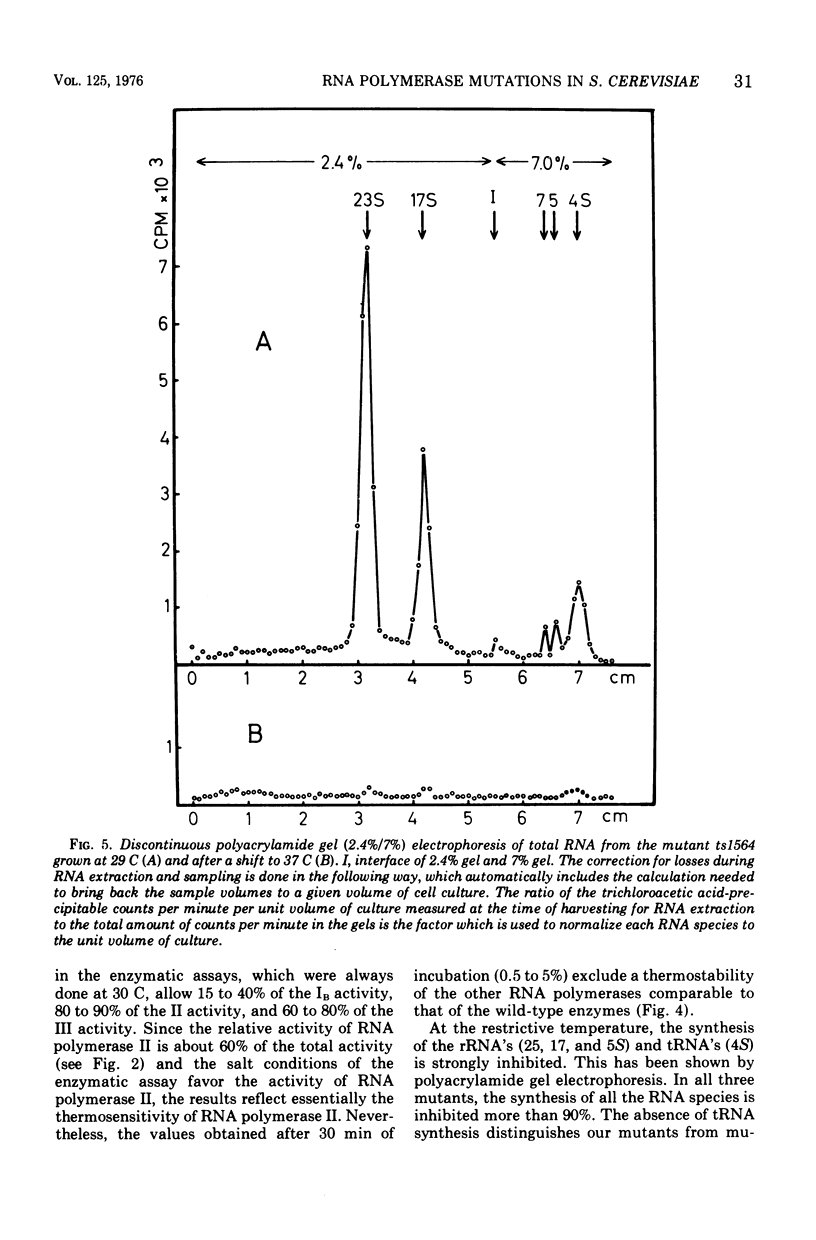
Abstract
Among 150 temperature-sensitive Saccharomyces cerevisiae mutants which we have isolated, 15 are specifically affected in ribonucleic acid (RNA) synthesis. Four of these mutants exhibit particularly drastic changes and were chosen for a more detailed study. In these four mutants, RNA synthesis is immediately blocked after a shift at the nonpermissive temperature (37 C), protein synthesis decays at a rate compatible with messenger RNA half-life, and deoxyribonucleic acid synthesis increases by about 40%. All the mutations display a recessive phenotype. The segregation of the four allelic pairs ts-/ts+ in diploids is mendelian, and the four mutants belong to three complementation groups. The elution patterns (diethylaminoethyl-Sephadex) of the three RNA polymerases of the mutants grown at 37 C for 3.5 h show very low residual activities. The in vitro thermodenaturation confirms the in vivo results; the half-lives of the mutant activities at 45 C are 10 times smaller than those of the wild-type enzymes. Polyacrylamide gel electrophoresis shows that the synthesis of all species of RNA is thermosensitive. The existence of three distinct genes, which are each indispensable for the activity of the three RNA polymerases in vivo as well as in vitro, strongly favors the hypothesis of three common subunits in the three RNA polymerases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman R., Schultz L. D., Hall B. D. Transcription in yeast: separation and properties of multiple FNA polymerases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1702–1706. doi: 10.1073/pnas.69.7.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler J. M., Sentenac A., Fromageot P. Isolation, structure, and general properties of yeast ribonucleic acid polymerase A (or I). J Biol Chem. 1974 Sep 25;249(18):5963–5970. [PubMed] [Google Scholar]
- Cano F. R., Kuo S. C., Lampen J. O. Lomofungin, an inhibitor of deoxyribonucleic acid-dependent ribonucleic acid polymerases. Antimicrob Agents Chemother. 1973 Jun;3(6):723–728. doi: 10.1128/aac.3.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer J. H., Sebastian J., Rownd R. H., Halvorson H. O. Transcription of Saccharomyces cerevisiae ribosomal DNA in vivo and in vitro. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2188–2192. doi: 10.1073/pnas.71.6.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezélée S., Sentenac A. Role of DNA-RNA hybrids in eukaryotes. Purification and properties of yeast RNA polymerase B. Eur J Biochem. 1973 Apr 2;34(1):41–52. doi: 10.1111/j.1432-1033.1973.tb02726.x. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A., Dabeva M. D., Mackedonski V. V. The action of alpha-amanitin in vivo on the synthesis and maturation of mouse liver ribonucleic acids. Biochem J. 1974 Mar;138(3):321–334. doi: 10.1042/bj1380321a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S., Warner J. R. Identification of ten genes that control ribosome formation in yeast. Mol Gen Genet. 1970;109(1):42–56. doi: 10.1007/BF00334045. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A., Sebastian J., Halvorson H. O. Yeast nuclear RNA polymerases I and II are immunologically related. Nat New Biol. 1973 Nov 21;246(151):73–74. doi: 10.1038/newbio246073a0. [DOI] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H., McLaughlin C. S. Temperature-sensitive yeast mutant defective in ribonucleic acid production. J Bacteriol. 1969 Sep;99(3):807–814. doi: 10.1128/jb.99.3.807-814.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegren G., Hwang Y. L., Oshima Y., Lindegren C. C. Genetical mutants induced by ethyl methanesulfonate in Saccharomyces. Can J Genet Cytol. 1965 Sep;7(3):491–499. doi: 10.1139/g65-064. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. H. Discontinuous polyacrylamide gel electrophoresis of RNA. Anal Biochem. 1972 Aug;48(2):442–447. doi: 10.1016/0003-2697(72)90097-8. [DOI] [PubMed] [Google Scholar]
- Ponta H., Ponta U., Wintersberger E. Purification and properties of DNA-dependent RNA polymerases from yeast. Eur J Biochem. 1972 Aug 18;29(1):110–118. doi: 10.1111/j.1432-1033.1972.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Romero J. Properties of a slow-sedimenting RNA synthesized by broadbean leaf tissue infected with broadbean mottle virus. Virology. 1973 Sep;55(1):224–230. doi: 10.1016/s0042-6822(73)81025-6. [DOI] [PubMed] [Google Scholar]
- Rosset R., Monier R. Instabilité de la séquence 3'-hydroxyle terminale du RNA de transfert chez les microorganismes. II. Etude du renouvellement de l'AMP terminal chez Escherichia coli en fonction du taux de croissance. Biochim Biophys Acta. 1965 Nov 8;108(3):385–393. [PubMed] [Google Scholar]
- Thouvenot D. R., Bourgeois C. M. Optimisation de la sélection de mutants de Saccharomyces cerevisiae par la nystatine. Ann Inst Pasteur (Paris) 1971 May;120(5):617–625. [PubMed] [Google Scholar]
- Tonnesen T., Friesen J. D. Inhibitors of ribonucleic acid synthesis in Saccharomyces cerevisiae: decay rate of messenger ribonucleic acid. J Bacteriol. 1973 Sep;115(3):889–896. doi: 10.1128/jb.115.3.889-896.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Roeder R. G. Role of DNA-dependent RNA polymerase 3 in the transcription of the tRNA and 5S RNA genes. Proc Natl Acad Sci U S A. 1974 May;71(5):1790–1794. doi: 10.1073/pnas.71.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]


