Abstract
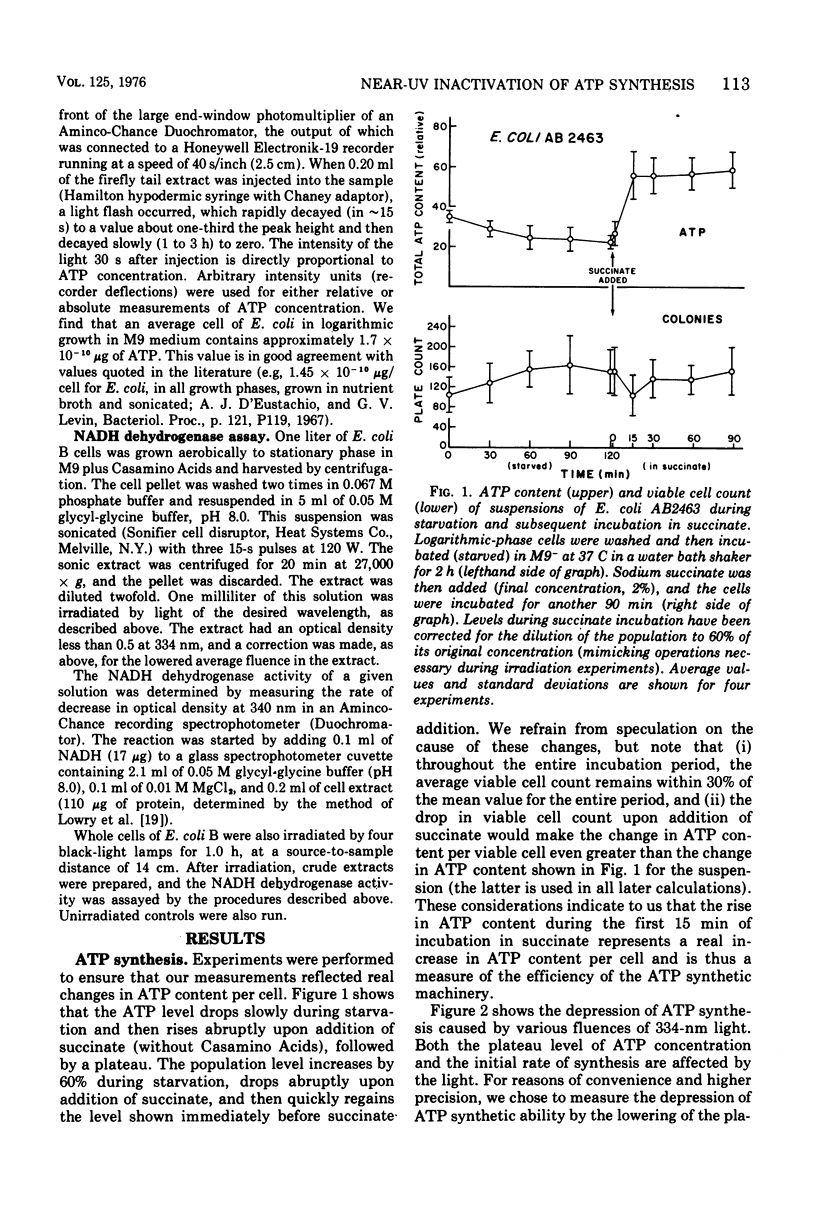
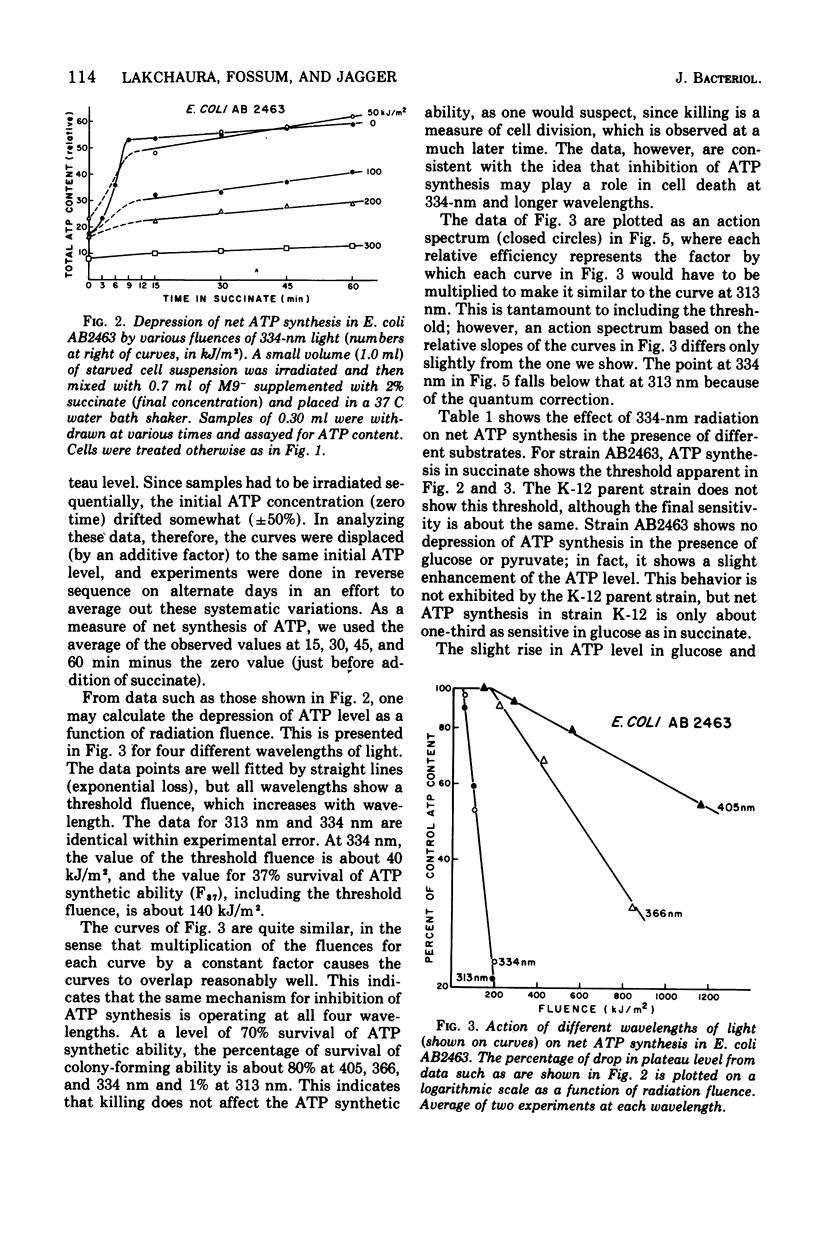
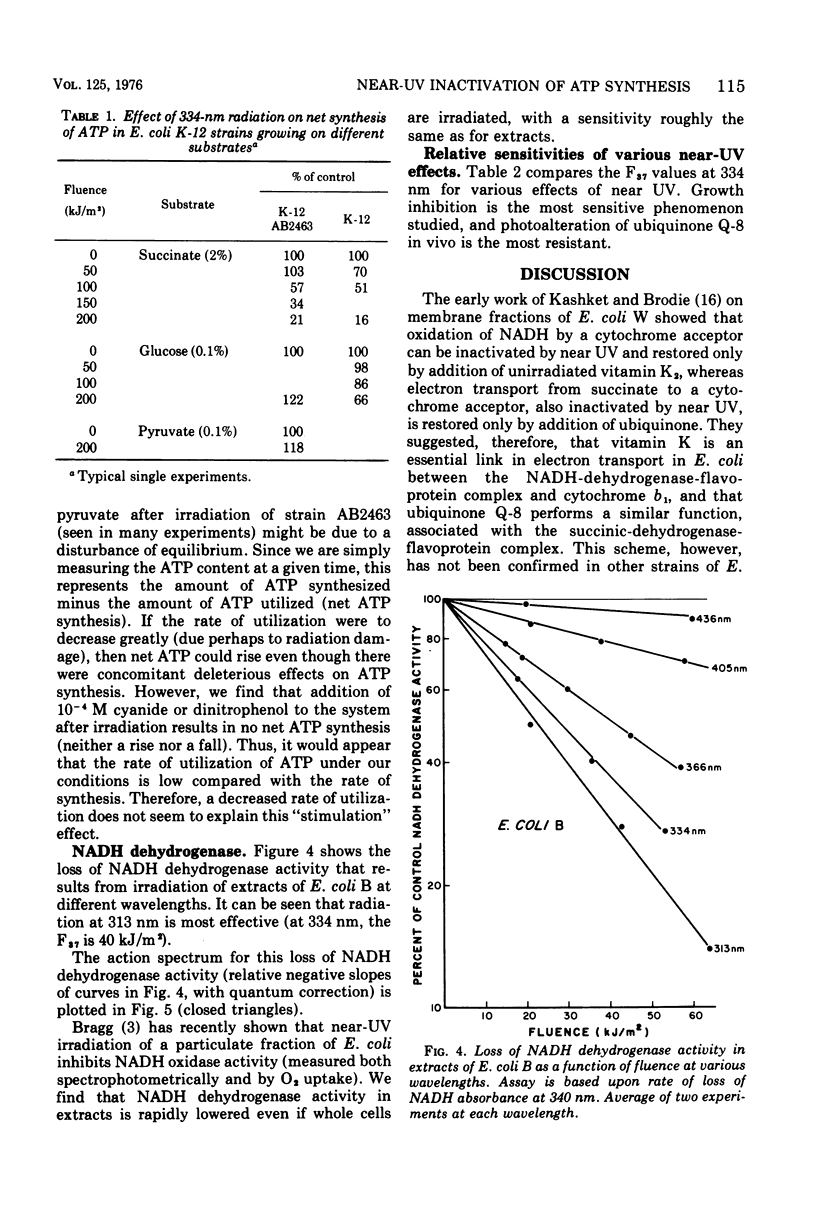
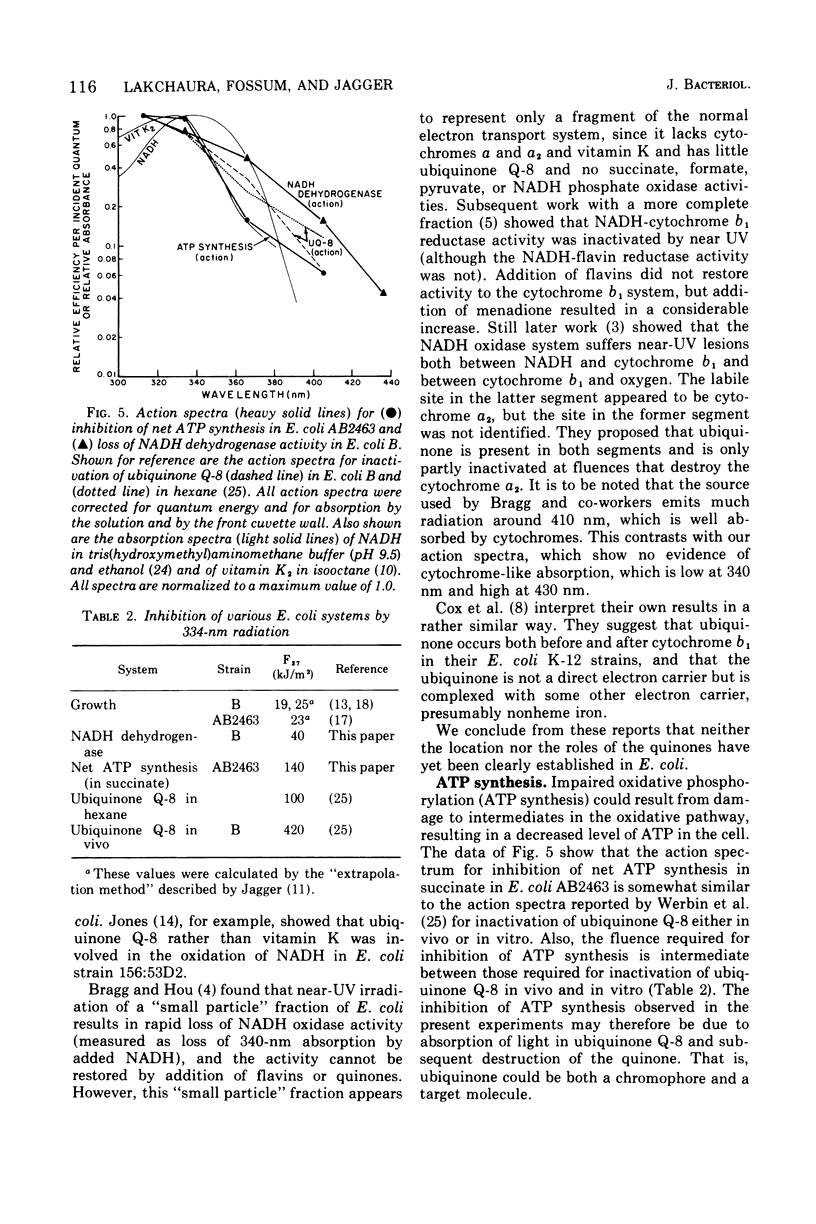
Near-ultraviolet (near-UV) light (300 to 380 nm) is a significant component of sunlight and has a variety of effects on biological systems. The present work is an attempt to identify chromophores (molecular absorbers of light) and targets (critical damaged molecules) for inhibition of adenosine triphosphate (ATP) synthesis in Escherichia coli by near UV. The fluence of 334 nm required for 37% survival of net ATP synthesis (F37) in E. coli AB2463 in succinate medium is 140 kJ/m2. The action spectrum for this inactivation is almost structureless, exhibiting a smooth transition from high efficiency at 313 nm to low efficiency at 405 nm. The action spectrum for inhibition of net ATP synthesis is consistent with the chromophore being either ubiquinone Q-8 or vitamin K2. The fluence required is consistent with ubiquinone Q-8 also being a target molecule. The activity of reduced nicotinamide adenine dinucleotide dehydrogenase in extracts of E. coli B is also inactivated by near UV and shows an F37 of about 40 kJ/m2. The action spectrum for this effect is quite structureless; it shows high efficiency at 313 nm and low efficiency at 435 nm. The data do not suggest a target molecule for this action, although it is possible that ubiquinone Q-8 absorbs the near-UV energy and then passes it on to some other target molecule. The data further indicate that inactivation of the oxidative phosphorylation system is not a primary factor in near-UV-induced growth delay in E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE A. F., BALLANTINE J. Oxidative phosphorylation in fractionated bacterial systems. II. The role of vitamin K. J Biol Chem. 1960 Jan;235:226–231. [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barran L. R., Daoust J. Y., Labelle J. L., Martin W. G., Schneider H. Differential effects of visible light on active transport in E. coli. Biochem Biophys Res Commun. 1974 Jan 23;56(2):522–528. doi: 10.1016/0006-291x(74)90874-2. [DOI] [PubMed] [Google Scholar]
- Bragg P. D. Effect of near-ultraviolet light on the respiratory chain of Escherichia coli. Can J Biochem. 1971 May;49(5):492–495. doi: 10.1139/o71-073. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Oxidative phosphorylation in Escherichia coli. Can J Biochem. 1968 Jul;46(7):631–641. doi: 10.1139/o68-099. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reduced nicotinamide adenine dinucleotide oxidation in Escherichia coli particles. I. Properties and cleavage of the electron transport chain. Arch Biochem Biophys. 1967 Mar;119(1):194–201. doi: 10.1016/0003-9861(67)90446-8. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P. Repression of oxidative phosphorylation in Escherichia coli B by growth in glucose and other carbohydrates. Biochem Biophys Res Commun. 1970 Oct 9;41(1):9–15. doi: 10.1016/0006-291x(70)90461-4. [DOI] [PubMed] [Google Scholar]
- Jagger J., Fossum T., McCaul S. Ultraviolet irradiation of suspensions of micro-organisms: possible errors involved in the estimation of average fluence per cell. Photochem Photobiol. 1975 May;21(5):379–382. doi: 10.1111/j.1751-1097.1975.tb06690.x. [DOI] [PubMed] [Google Scholar]
- Jagger J. Growth delay and photoprotection induced by near-ultraviolet light. Res Prog Org Biol Med Chem. 1972;3(Pt 1):383–401. [PubMed] [Google Scholar]
- Jagger J. Inhibition by sunlight of the growth of Escherichia coli B/r. Photochem Photobiol. 1975 Jul-Aug;22(1-2):67–70. doi: 10.1111/j.1751-1097.1975.tb06724.x. [DOI] [PubMed] [Google Scholar]
- Jones R. G. Ubiquinone deficiency in an auxotroph of Escherichia coli requiring 4-hydroxybenzoic acid. Biochem J. 1967 Jun;103(3):714–719. doi: 10.1042/bj1030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHKET E. R., BRODIE A. F. Effects of near-ultraviolet irradiation on growth and oxidative metabolism of bacteria. J Bacteriol. 1962 May;83:1094–1100. doi: 10.1128/jb.83.5.1094-1100.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHKET E. R., BRODIE A. F. Oxidative phosphorylation in fractionated bacterial systems. X. Different roles for the natural quinones of Escherichia coli W in oxidative metabolism. J Biol Chem. 1963 Jul;238:2564–2570. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakchaura B. D., Jagger J. Induction by cyanide, dinitrophenol, and trinitrophenol of growth inhibition and of recovery from far-ultraviolet killing in Escherichia coli B: relationship to photoprotection. Radiat Res. 1972 Mar;49(3):631–646. [PubMed] [Google Scholar]
- Lakchaura B. D. Photoprotection from killing in ultraviolet-sensitive Escherichia coli K-12 mutants: involvement of excision-resynthesis repair. Photochem Photobiol. 1972 Sep;16(3):197–202. doi: 10.1111/j.1751-1097.1972.tb06291.x. [DOI] [PubMed] [Google Scholar]
- Ramabhadran T. V. Effects of near-ultraviolet and violet radiations (313-405 nm) on DNA, RNA, and protein synthesis in E. coli B/r: implications for growth delay. Photochem Photobiol. 1975 Sep-Oct;22(3-4):117–123. doi: 10.1111/j.1751-1097.1975.tb08822.x. [DOI] [PubMed] [Google Scholar]
- Ramírez J., Smith L. Synthesis of adenosine triphosphate in intact cells of Rhodospirillum rubrum and Rhodopseudomonas spheroides on oxygenation or illumination. Biochim Biophys Acta. 1968 Feb 12;153(2):466–475. doi: 10.1016/0005-2728(68)90088-1. [DOI] [PubMed] [Google Scholar]
- Rupert C. S. Dosimetric concepts in photobiology. Photochem Photobiol. 1974 Sep;20(3):203–212. doi: 10.1111/j.1751-1097.1974.tb06568.x. [DOI] [PubMed] [Google Scholar]
- SIEGEL J. M., MONTGOMERY G. A., BOCK R. M. Ultraviolet absorption spectra of DPN and analogs of DPN. Arch Biochem Biophys. 1959 Jun;82(2):288–299. doi: 10.1016/0003-9861(59)90124-9. [DOI] [PubMed] [Google Scholar]
- Werbin H., Lakchaura B. D., Jagger J. Near-ultraviolet modification of Escherichia coli B ubiquinone in vivo and in vitro. Photochem Photobiol. 1974 May;19(5):321–328. doi: 10.1111/j.1751-1097.1974.tb06519.x. [DOI] [PubMed] [Google Scholar]


