Abstract
A novel multispecific organic anion transporting polypeptide (oatp2) has been isolated from rat brain. The cloned cDNA contains 3,640 bp. The coding region extends over 1,983 nucleotides, thus encoding a polypeptide of 661 amino acids. Oatp2 is homologous to other members of the oatp gene family of membrane transporters with 12 predicted transmembrane domains, five potential glycosylation, and six potential protein kinase C phosphorylation sites. In functional expression studies in Xenopus laevis oocytes, oatp2 mediated uptake of the bile acids taurocholate (Km ≈ 35 μM) and cholate (Km ≈ 46 μM), the estrogen conjugates 17β-estradiol-glucuronide (Km ≈ 3 μM) and estrone-3-sulfate (Km ≈ 11 μM), and the cardiac gylcosides ouabain (Km ≈ 470 μM) and digoxin (Km ≈ 0.24 μM). Although most of the tested compounds are common substrates of several oatp-related transporters, high-affinity uptake of digoxin is a unique feature of the newly cloned oatp2. On the basis of Northern blot analysis under high-stringency conditions, oatp2 is highly expressed in brain, liver, and kidney but not in heart, spleen, lung, skeletal muscle, and testes. These results provide further support for the overall significance of oatps as a new family of multispecific organic anion transporters. They indicate that oatp2 may play an especially important role in the brain accumulation and toxicity of digoxin and in the hepatobiliary and renal excretion of cardiac glycosides from the body.
Keywords: liver transport, brain transport, bile acids, estrogen conjugates, digoxin
Recently, sodium-independent organic anion transporting polypeptides have been cloned from rat (oatp) and human (OATP) livers (1, 2). Oatp, which now is called oatp1, represents an 80-kDa basolateral (sinusoidal) transporter (3) that can mediate hepatocellular uptake of a wide range of amphipathic substrates including bromosulfophthalein, bile acids, estrogen conjugates, neutral steroids, certain organic cations (e.g., N-propylajmalinium), peptidomimetic drugs, and the mycotoxin ochratoxin A (4–6). On Northern blots, the oatp1 cDNA reacted with different mRNAs in various organs (1). By using sequence specific antibodies, oatp1 could be localized also at the apical portion of the S3 segment of the proximal tubule of rat kidney (3) and at the apical surface of the choroid plexus of rat brain (7). In addition, oatp1-related transporters have been isolated and include the basolateral methotrexate transporter OAT-K1 of the rat kidney (8) and the prostaglandin transporter (9).
The human OATP exhibits a 67% amino acid sequence identity with the rat oatp1 (2). Furthermore, in comparative functional expression studies the affinities and transport activities for selected substrates were partly different between OATP and oatp1 (10). The most distinct feature of OATP as compared with oatp1, however, was its markedly higher reactivity with total brain mRNA, suggesting additional high-level expression of OATP (or closely related polypeptides) in human brain regions other than just the choroid plexus (2). These observations indicated that OATP is not encoded by the homologous oatp1 gene in humans but actually represents a new member of the oatp gene family of membrane transporters. On the basis of this hypothesis, we used a specific OATP cDNA oligonucleotide to identify, isolate, and characterize a new oatp1-related transporter from rat brain. A unique feature of this so called oatp2 is its high affinity and transport activity for the cardiovascular drug digoxin, suggesting that it may play a role in the pathogenesis of toxic central nervous system effects in cardiac glycoside overdose.
MATERIALS AND METHODS
Materials.
[3H]Taurocholic acid (3.47 Ci/mmol; 1 Ci = 37 GBq), [3H]cholic acid (27.5 Ci/mmol), [3H]estrone-3-sulfate (50.9 Ci/mmol), 17β-[3H]-estradiol-d-glucuronide (49 Ci/mmol), [3H]ouabain (20.5 Ci/mmol), and [3H]digoxin (15 Ci/mmol) were obtained from Du Pont-New England Nuclear.
Animals.
Mature Xenopus laevis females were purchased from the University of Konstanz (Konstanz, Germany) and kept under standard conditions as described (11). Male Sprague–Dawley rats (SUT:SDT) were obtained from the Institut für Labortierkunde, University of Zürich (Zürich, Switzerland).
Isolation of mRNA.
Total rat brain and rat liver RNA was prepared as described (12). The mRNA was isolated with the PolyATtract mRNA isolation system (Promega).
Human OATP Oligonucleotide Probe.
A 60-bp OATP oligonucleotide probe (called HOT 584) was used to screen a rat brain cDNA library. The antisense probe included nucleotides 584–525 of OATP cDNA and had the following sequence 5′-AGGCAGGATGGGAGTTTCACCCATTCCACGTACAATATTGCCTACTAGGACGTACACCCA-3′.
Construction and Screening of a Rat Brain cDNA Library.
Rat brain mRNA was size-fractionated in a linear sucrose gradient (13). cDNA was synthesized from the 3.5- to 4.5-kb size class mRNA fraction by using the ZAP cDNA Gigapack III Gold cloning kit (Stratagene). The cDNA was ligated unidirectionally (EcoRI–XhoI) into the Uni-ZAP XR vector resulting in a library of 106 plaque-forming units/ml. Aliquots of the library were plated out at a density of 2.5 × 104 plaque-forming units per master plate. Replica filters (Hybond-N; Amersham) were screened with the specific HOT 584 probe (see above). The oligonucleotide was labeled with [α-32P]deoxycytidine 5′-triphosphate (Amersham; 3,000 Ci/mmol) by using terminal deoxynucleotidyltransferase (Promega). Prehybridization and hybridization were performed in standard hybridization solution (hybridization buffer tablets, Amersham). After 2 h of prehybridization at 60°C, the filters were transferred into fresh hybridization solution containing the radiolabeled oligonucleotide and hybridized for 12 h at 60°C. Thereafter, the filters were washed for two 15-min periods in 6× standard saline citrate (SSC)/0.1% SDS at 55°C. They were exposed to autoradiography film for several hours. After several rounds of screening, a single clone was isolated. The new rat brain oatp clone (called oatp2) was functionally tested in X. laevis oocytes (see below). After demonstration of its functional competence, oatp2 was sequenced with the help of the T7 sequencing kit (Pharmacia P-L Biochemicals) in both directions by using custom synthesized oligonucleotide primers. Sequence analysis was performed as described (1).
Transport Assays in Oocytes.
cRNA (derived from the cloned cDNA) was transcribed in vitro from rat oatp2 cDNA by using T3 RNA polymerase (Promega). X. laevis oocytes were prepared as described (13). After an overnight incubation at 18°C, healthy oocytes were microinjected with 5 ng of oatp2 cRNA and cultured for 3 days to allow the expression of oatp2 in the oocyte plasma membrane. Tracer uptake studies were performed in an Na+-free medium that consisted of 100 mM choline chloride, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 10 mM Hepes, adjusted to pH 7.5 with Tris. Ten to fifteen oocytes were prewashed in the incubation medium and then incubated at 25°C in 100 μl of the same medium containing different radiolabeled substrates. After the indicated time intervals, uptake of tracer was stopped, and oocytes were washed with 3 ml of ice-cold incubation buffer. Each oocyte was dissolved in 10% SDS. After addition of 5 ml of scintillation fluid (Ultima Gold; Canberra Packard, Zürich, Switzerland), the oocyte-associated radioactivity was measured in a Packard Tri-Carb 2200 CA liquid scintillation analyzer (Cranberra Packard).
Northern Blot Analysis.
High-stringency Northern blot analysis was performed as described in the corresponding figure legends.
RESULTS
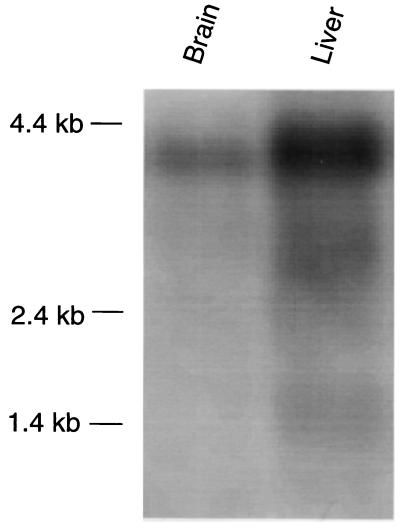
Because of the high expression of OATP in human brain (2), we first investigated whether an OATP analogue is also expressed in rat brain. As illustrated in Fig. 1, the specific OATP oligonucleotide HOT 584 reacted predominantly with a rat brain mRNA of ≈4 kb, whereas in rat liver three transcripts of ≈1.4 kb, ≈2.8 kb, and ≈4 kb were detected. On the basis of these results, a rat brain cDNA library was constructed from a 3.5- to 4.5-kb size class mRNA fraction. Repeated screenings of the library finally resulted in the isolation of the new rat brain oatp2 clone. Its total cDNA insert contained 3,640 nucleotides including 1,539 untranslated nucleotides at the 3′ end. Based on the Kosak consensus sequence (14), the initiation site was assigned to the first ATG codon at position 119. Consequently, the coding region of the cloned oatp2 cDNA extends over 1,983 nucleotides, thus predicting a polypeptide of 661 amino acids with a calculated molecular mass of ≈73 kDa. In Fig. 2, the deduced amino acid sequence of oatp2 is compared with previously cloned members of the oatp family of membrane transporters. Overall oatp2 exhibits an amino acid sequence identity of 77% to oatp1 and OAT-K1 and of 73% to the human OATP. The corresponding identity of oatp2 to the prostaglandin transporter (9) is 35%. On the basis of the Kyte–Doolittle hydropathy analysis (15), oatp2 has also 12 transmembrane spanning domains, thus indicating five potential N-linked glycosylation sites in the extracellular loops (Fig. 2). In addition, there are six potential protein kinase C phosphorylation sites (16) at positions 4, 383, 580, 596, 634, and 645 on intracellular protein domains. And finally, two overlapping putative Cys–Cys zinc-finger motifs, which first have been described in the assumed DNA-binding protein matrin F/G (17), the cloned precursor portion of the prostaglandin transporter (9), are still conserved in all oatp gene products between amino acids 438 and 487 (Fig. 2).
Figure 1.
Northern blot analysis of rat brain and rat liver mRNA with the human-OATP-specific oligonucleotide HOT 584. mRNA (2 μg) was isolated, denatured, and electrophoresed in a 1% agarose/formaldehyde gel. After transfer to a nylon membrane (Hybond N, Amersham), prehybridization was performed for 2 h at 60°C. The radiolabeled oligonucleotide HOT 584 was added and hybridization was performed overnight in standard hybridization solution. The blot was washed for two 15-min periods in 6× SSC/0.1% SDS at 55°C. The blot was exposed to autoradiography film for 3 days.
Figure 2.
Comparison of the deduced amino acid sequences between oatp2, oatp1, OATP, and OAT-K1. Putative membrane-spanning domains are indicated by lines and numbered M1 to M12. ★, Putative N-linked glycosylation sites of oatp2; ▪, potential protein kinase C phosphorylation sites of oatp2. Overlapping putative Cys–Cys zinc-finger motifs conserved in all oatps are boxed.
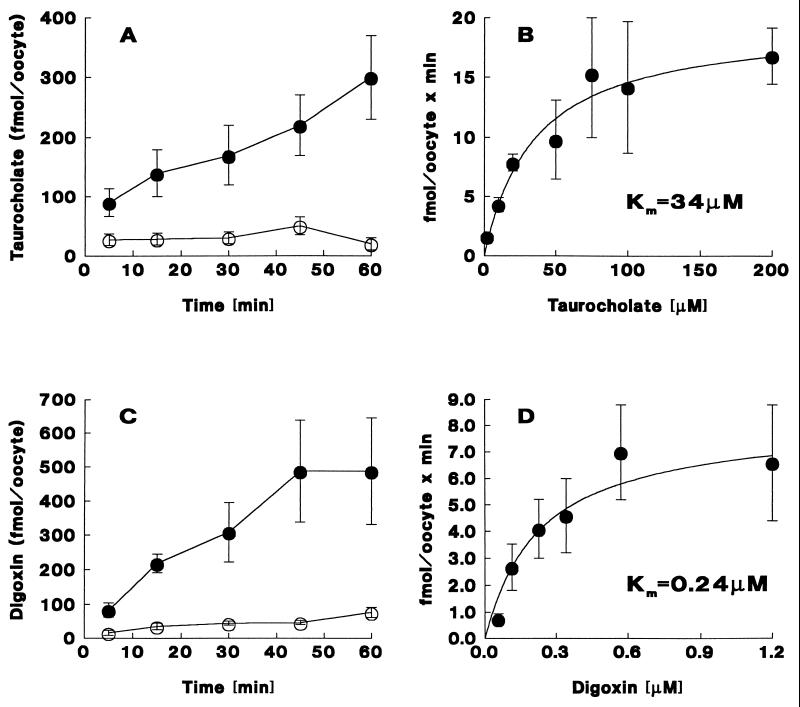
The transport function of oatp2 was investigated in cRNA-injected oocytes. The various candidate transport substrates were selected on the basis of the broad substrate specificity of oatp1 and OATP (1, 2, 4, 18, 19). As illustrated in Fig. 3, sodium-independent uptake of taurocholate increased linearly with time and was approximately 10-fold higher after 60 min in oatp2-cRNA-injected as compared with water-injected oocytes (Fig. 3A). In addition, oatp2-mediated taurocholate uptake exhibited saturation kinetics with an apparent Km of ≈35 μM (Fig. 3B and Table 1). Saturable oatp2-mediated uptake was also found for the characteristic oatp1 and OATP substrates cholate (Km ≈ 46 μM), 17β-estradiol-glucuronide (Km ≈ 3 μM), estrone-3-sulfate (Km ≈ 11 μM), and ouabain (Km ≈ 470 μM) (Table 1). In addition, and most importantly, oatp2 mediated unique high-affinity uptake of the cardiac glycoside digoxin. This oatp2-dependent digoxin uptake increased linearly during the initial 45 min and resulted in an ≈11-fold higher maximal digoxin accumulation in oatp2-cRNA-injected as compared with water-injected oocytes (Fig. 3C). Furthermore, saturation kinetics revealed an apparent Km of ≈0.24 μM for oatp2-mediated digoxin uptake (Fig. 3D). Hence, among all substrates tested, digoxin was taken up by oatp2 with the highest affinity (≈2,000-fold higher than ouabain; Table 1). Parenthetically, the oatp2-mediated digoxin uptake activity was ≈45-fold higher than the digoxin uptake signal observed in oocytes injected with total brain RNA (data not shown). Parallel experiments in the presence of NaCl (instead of choline chloride) revealed no Na+-dependent uptake portion for any of the oatp2 substrates tested. No oatp2-mediated transport was found for p-aminohippurate, pyruvate, glutamate, norepinephrine, epinephrine, serotonin, dopamine, corticosterone, and leukotriene C4 (data not shown).
Figure 3.
Oatp2-mediated taurocholate and digoxin transport in Xenopus laevis oocytes. (A) Time course of sodium-independent uptake of [3H]taurocholate (10 μM) in oatp2 cRNA (5 ng)-injected (•) and in water-injected (○) oocytes. (B) Saturation kinetics of initial (15 min) sodium-independent [3H]taurocholate uptake in oatp2 cRNA (5 ng)-injected oocytes. (C) Time course of sodium-independent uptake of [3H]digoxin (0.57 μM) in oatp2 cRNA (5 ng)-injected (•) and in water-injected (○) oocytes. (D) Saturation kinetics of initial (15 min) sodium-independent [3H]digoxin uptake in oatp2 cRNA (5 ng)-injected oocytes. In the kinetic uptake curves (B and D), nonspecific taurocholate and digoxin uptakes into water-injected oocytes were subtracted from total uptake values. Individual data points represent the mean ± SD of 10–15 oocyte uptake measurements. The curves were fitted by computerized nonlinear regression analysis (systat).
Table 1.
Comparison of substrate Km values for various members of the oatp gene family of membrane transporters, as determined in cRNA-injected Xenopus laevis oocytes
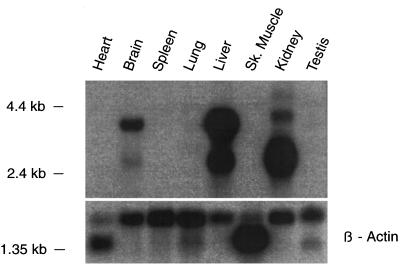
Finally, the tissue distribution of oatp2 mRNA was investigated by Northern blot analysis under high-stringency conditions. As illustrated in Fig. 4, the 5′ oatp2 cDNA probe (nucleotides 1–360) reacted predominantly with a ≈4-kb size class mRNA of rat brain, which most probably corresponds to the original brain mRNA detected with the human OATP oligonucleotide HOT 584 (Fig. 1). Two highly reactive mRNAs of ≈4 kb and ≈2.8 kb were also found in liver and kidney, whereas no hybridization signals were found in heart, spleen, lung, skeletal muscle, and testes (Fig. 4). Whereas the 4-kb mRNA in liver and kidney most probably corresponds to the full-length transcript of oatp2, the 2.8-kb mRNA may contain a shorter untranslated region at the 3′ end. Hence, both reactive liver and kidney mRNAs could still encode the same protein, indicating that oatp2 is even higher expressed in liver and kidney than in brain. In separate Northern blots, the species distribution of oatp2 was investigated under low-stringency conditions (i.e., overnight hybridization at 37°C, 30% formamide; washing with 2× SSC/0.1% SDS at 50°C for 30 min). Positive reactions were only obtained with mRNA of rat and mouse livers but not with mRNA of skate, frog, and turtle liver (data not shown), suggesting that oatp2 is probably not present in the liver of low vertebrate animals.
Figure 4.
Northern blot analysis for oatp2 mRNA in various rat tissues. The multiple tissue Northern blot containing 2 μg from various rat tissues was purchased from CLONTECH. The blot was prehybridized for 2 h at 42°C in 50% formamide/5× SSPE (SSPE = 0.18 M NaCl/10 mM sodium phosphate, pH 7.4/1 mM EDTA)/5× Denhardt’s solution/0.2% SDS/denatured salmon sperm DNA (100 μg/ml). After overnight hybridization with a PCR-amplified 32P-labeled oatp2 cDNA probe (nucleotides 1–360), the blot was washed for two 5-min periods in 2× SSC/0.1% SDS at room temperature and for 30 min in 0.1× SSC/0.1% SDS at 65°C. Subsequently, the blot was exposed to autoradiography film for 1 day. A human β-actin probe served as a control. The different sizes of β-actin transcripts are due to the detection of various isoforms of β-actin mRNAs, as specified by the supplier of the multiple tissue Northern blot.
DISCUSSION
We have isolated a new member of the oatp gene family of membrane transporters from rat brain. Although the isolated oatp2 cDNA is larger (3,640 bp) than the previously cloned oatp gene products oatp1 (2,759 bp) (1), OATP (2,737 bp) (2), and OAT-K1 (2,788 bp) (8), the ORFs are of similar size and encode proteins with 661, 670, 670, and 669 amino acids, respectively. These results indicate that the previously observed heterogenous reaction patterns of oatp1 and OATP cDNAs with differently sized mRNAs from various organs (1, 2) resulted from variable sizes of the 3′ untranslated regions rather than from different lengths of the ORFs. However, it cannot be excluded at present that differently sized additional members of the oatp gene family still remain to be characterized in various organs. All oatps cloned so far are homologous proteins with 12 putative membrane-spanning domains and similar potential extracellular glycosylation and intracellular phosphorylation sites (Fig. 2). The presence of an overlapping putative DNA-binding Cys–Cys zinc-finger motif (Fig. 2) is unique and certainly not characteristic for a membrane transporting polypeptide. Its functional significance, if any, remains to be elucidated. Interestingly, however, similar Cys–Cys zinc-finger motifs have been described for steroid receptors (20). Furthermore, some plasma-membrane-associated proteins have recently been shown to reside also within the cell nucleus (21), raising the possibility of a similar dual residence of oatp2 or oatp2-related proteins as well.
Functionally, the isolated oatp2 mediates multispecific substrate transport as previously described for oatp1 (1, 4, 22) and OATP (2, 19). However, although the Km values of oatp2 for the tested bile acids and estrogen conjugates are similar to oatp1, the affinity for ouabain is approximately 4- and 12-fold higher than with oatp1 and OATP, respectively (Fig. 3 and Table 1). This higher ouabain affinity of oatp2 is closer to the Km values of 127–348 μM reported for ouabain uptake into rat hepatocytes (for review see ref. 10). Since oatp2 is also highly expressed in rat liver (Fig. 4), these results suggest that oatp2 may play a more important role than oatp1 in the uptake and clearance of ouabain by rat liver. A unique high-affinity substrate of oatp2 is digoxin (Fig. 3 and Table 1), which represents a traditional and still important cardiovascular drug. Its elimination from the body occurs by the following pathways: approximately 25% by hepatobiliary excretion into bile, 15% via the gut mucosa, and about 60% via the urine (23). Furthermore, digoxin overdose is well known to induce central nervous system symptoms including fatigue, anorexia, vomiting, visual disturbances, headache, and confusion (24). Interestingly, it has also been shown that digoxin markedly accumulates in the brain of mdr1a knock-out mice even in the presence of very low digoxin concentrations in plasma, suggesting the presence of active (i.e., concentrative) digoxin uptake mechanisms in certain brain cells (23). The present observations indicate that oatp2, in conjunction with the mdr1a efflux pump (25), might also be involved in the brain accumulation and central nervous system toxicity of digoxin. This assumption, however, requires more definite studies in regard to the driving force for oatp2-mediated transmembrane substrate transport and the exact topical and cellular localization of oatp2 in rat brain. In addition, the major endogenous substrate of oatp2 remains unknown. Obviously, endogenous digoxin- and ouabain-like factors (26, 27) would be logical candidates once their exact chemical identity and physiological functions has been more definitely characterized.
In conclusion, the present study adds a new member to the growing list of the oatp gene family of membrane transporters. Besides the close structural and functional similarities to the previously isolated oatps, the cloned oatp2 mediates unique high-affinity transport of the cardiac glycoside digoxin. Its high-level expression in brain, liver, and possibly kidney (Fig. 4) indicate that oatp2 may play an important role in the accumulation of digoxin in brain tissue and in the hepatobiliary and renal excretion of digoxin from the body.
Acknowledgments
This study was supported by the Swiss National Science Foundation (Grants 31.45677.95 to B.H. and 31.045536.95 to P.J.M.) and the Cloëtta Foundation, Zurich. B.N. was a recipient of a postdoctoral research fellowship award from the Deutsche Forschungsgemeinschaft (Bonn, Germany).
ABBREVIATIONS
- OATP
human organic anion transporting polypeptide
- oatp1
original rat liver organic anion transporting polypeptide
- oatp2
organic anion transporting polypeptide, as cloned from rat brain in this study
- OAT-K1
organic anion transporter of rat kidney
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. U88036).
References
- 1.Jacquemin E, Hagenbuch B, Stieger B, Wolkoff A W, Meier P J. Proc Natl Acad Sci USA. 1994;91:133–137. doi: 10.1073/pnas.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kullak-Ublick G A, Hagenbuch B, Stieger B, Schteingart C D, Hofmann A F, Wolkoff A W, Meier P J. Gastroenterology. 1995;109:1274–1282. doi: 10.1016/0016-5085(95)90588-x. [DOI] [PubMed] [Google Scholar]
- 3.Bergwerk A J, Shi X, Ford A C, Kanai N, Jacquemin E, Burk R D, Bai S, Novikoff P M, Stieger B, Meier P J, Schuster V L, Wolkoff A W. Am J Physiol. 1996;271:G231–G238. doi: 10.1152/ajpgi.1996.271.2.G231. [DOI] [PubMed] [Google Scholar]
- 4.Bossuyt X, Müller M, Hagenbuch B, Meier P J. J Pharmacol Exp Ther. 1996;276:891–896. [PubMed] [Google Scholar]
- 5.Eckhardt U, Horz J A, Petzinger E, Stüber W, Reers M, Dickneite G, Daniel H, Wagener M, Hagenbuch B, Stieger B, Meier P J. Hepatology. 1996;24:380–384. doi: 10.1002/hep.510240215. [DOI] [PubMed] [Google Scholar]
- 6.Kontaxi M, Eckhardt U, Hagenbuch B, Stieger B, Meier P J, Petzinger E. J Pharmacol Exp Ther. 1996;279:1507–1513. [PubMed] [Google Scholar]
- 7.Angeletti R H, Novikoff P M, Juvvadi S R, Fritschy J M, Meier P J, Wolkoff A W. Proc Natl Acad Sci USA. 1997;94:283–286. doi: 10.1073/pnas.94.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito H, Masuda S, Inui K. J Biol Chem. 1996;271:20719–20725. doi: 10.1074/jbc.271.34.20719. [DOI] [PubMed] [Google Scholar]
- 9.Kanai N, Lu R, Satriano J A, Bao Y, Wolkoff A W, Schuster V L. Science. 1995;268:866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- 10.Meier, P. J., Eckhardt, U., Schroeder, A., Hagenbuch, B. & Stieger, B. (1997) Hepatology, in press. [DOI] [PubMed]
- 11.Colman A. In: Transcription and Translation: A Practical Approach. Hames B D, Higgin S, editors. Oxford: IRL; 1986. pp. pp.271–302. [Google Scholar]
- 12.Chromczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 13.Hagenbuch B, Scharschmidt B F, Meier P J. Biochem J. 1996;316:901–904. doi: 10.1042/bj3160901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosak M. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyte J, Doolittle R F. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 16.Kennelly P J, Krebs G D. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- 17.Hakes D J, Berezney R. Proc Natl Acad Sci USA. 1991;88:6186–6190. doi: 10.1073/pnas.88.14.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullak-Ublick G A, Hagenbuch B, Stieger B, Wolkoff A W, Meier P J. Hepatology. 1994;20:411–416. [PubMed] [Google Scholar]
- 19.Bossuyt X, Müller M, Meier P J. J Hepatol. 1996;25:733–738. doi: 10.1016/s0168-8278(96)80246-7. [DOI] [PubMed] [Google Scholar]
- 20.Freedman L P. Endocr Rev. 1992;13:129–145. doi: 10.1210/edrv-13-2-129. [DOI] [PubMed] [Google Scholar]
- 21.Keon B H, Schäfer St, Kuhn C, Grund Ch, Franke W W. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanai N, Lu R, Bao Y, Wolkoff A W, Vore M, Schuster V L. Am J Physiol. 1996;270:F326–F331. doi: 10.1152/ajprenal.1996.270.2.F326. [DOI] [PubMed] [Google Scholar]
- 23.Mayer U, Wagenaar E, Beijnen J H, Smit J W, Meijer D K F, van Asperen J, Borst P, Schinkel A H. Br J Pharmacol. 1996;119:1038–1044. doi: 10.1111/j.1476-5381.1996.tb15775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly R A, Smith T W. Am J Cardiol. 1992;69:108G–119G. doi: 10.1016/0002-9149(92)91259-7. [DOI] [PubMed] [Google Scholar]
- 25.Schinkel A H, Wagenaar E, van Deemter L, Mol C A A M, Borst P. J Clin Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qazzaz H M A M, Goudy S L, Valdes R. J Biol Chem. 1996;271:8731–8737. doi: 10.1074/jbc.271.15.8731. [DOI] [PubMed] [Google Scholar]
- 27.Ludens J H, Clark M A, DuCharme D W, Harris D W, Lutzke B S, Mandel F, Mathews W R, Sutter D M, Hamlyn J M. Hypertension. 1991;17:923–929. doi: 10.1161/01.hyp.17.6.923. [DOI] [PubMed] [Google Scholar]