Abstract
To address genetic influences on hippocampal neurogenesis in adult mice, we compared C57BL/6, BALB/c, CD1(ICR), and 129Sv/J mice to examine proliferation, survival, and differentiation of newborn cells in the dentate gyrus. Proliferation was highest in C57BL/6; the survival rate of newborn cells was highest in CD1. In all strains ≈60% of surviving newborn cells had a neuronal phenotype, but 129/SvJ produced more astrocytes. Over 6 days C57BL/6 produced 0.36% of their total granule cell number of 239,000 as new neurons, BALB/c 0.30% of 242,000, CD1 (ICR) 0.32% of 351,000, and 129/SvJ 0.16% of 280,000. These results show that different aspects of adult hippocampal neurogenesis are differentially influenced by the genetic background.
The birth of new neurons in the hippocampus of adult rodents has challenged the old dogma that there is no neurogenesis in the adult brain. Proliferating neuronal stem or progenitor cells have been shown to exist in the subgranular layer of the dentate gyrus (1). The neuronal character of the newborn cells has been confirmed ultrastructurally (2) by means of retrograde labeling of their neurites (3) and with immunohistochemical markers, including neuron-specific enolase (4), the neuronal nuclear protein NeuN, and granule cell marker calbindin D28k (5). Despite these efforts, little is known about the terminal fate of these cells, the functional relevance of their connections, and the regulation of their development.
Factors that have been shown to have a regulatory effect on adult hippocampal neurogenesis are age, hormonal status, excitatory input, and several growth factors (5–7).
Studies of song birds were the first to suggest that adult neurogenesis could be functionally regulated. In canaries, a high neuronal turnover was noted in the higher vocal center involved in song learning (8), and in the hippocampus of adult chickadees more new neurons were recruited in seasons in which the birds occupied a larger territory and were thus challenged to develop an increased spatial memory (9, 10). We have recently shown that the dentate gyrus of adult mice that lived in an enriched environment had a greater number of granule cell neurons than in control littermates (11). Proliferation of progenitor cells was not influenced, but the survival of the newborn cells was increased by 60%. This result demonstrated that in the adult mammalian brain, neuroanatomical plasticity is possible on the level of neuronal cell numbers. Thus, although environmental stimuli can influence adult neurogenesis, little is known about the underlying genetic determinants.
We have addressed genetic background in the regulation of adult hippocampal neurogenesis by examining strain differences in animals that were held under equivalent housing conditions. We used adult mice from four commonly used laboratory strains: C57BL/6, BALB/c, CD1 (ICR), and 129/SvJ.
Proliferation of progenitor cells in situ was assessed by labeling dividing cells by systemic application of the thymidine analog 5-bromodeoxyuridine (BrdU), which is incorporated into the DNA of dividing cells in the S phase of the mitotic cycle (12) and can be detected immunohistochemically. Survival of the cells was addressed by comparing the numbers of BrdU-positive cells 1 day and 4 weeks after the last injection. All cell counts were performed using unbiased stereological counting techniques (13, 14). Neuronal and glial differentiation were examined by immunohistochemistry and confocal microscopy with antibodies to BrdU, and neuronal and glial markers.
MATERIALS AND METHODS
Animals.
For morphological studies a total of 40 female mice were used, 10 per strain. Nine-week-old C57BL/6, BALB/c, and CD1 (ICR) mice were obtained from Harlan Sprague–Dawley, and 8-week-old 129/SvJ mice were from The Jackson Laboratory. Animals were transported under similar conditions and were held according to the standard National Institutes of Health regulations in regular laboratory cages, three or four mice per cage. They were allowed 48 h of rest before the start of the experiment.
BrdU Injections.
BrdU (Sigma) was dissolved in 0.9% NaCl and filtered at 22 μm. The animals received i.p. injections of 50 μg/g body weight, one per day for 6 days.
Tissue Preparation.
Animals were perfused transcardially with 4% paraformaldehyde in phosphate buffer. The brains were removed, stored in fixative overnight, and transferred into 30% sucrose. Sections (40 μm) were cut coronally on a sliding microtome and stored at −20°C in cryoprotectant containing 25% ethylene glycol, 25% glycerin, and 0.05 M phosphate buffer. Primary and secondary antibodies were diluted in Tris-buffered saline containing 0.1% Triton X-100 and 3% horse or donkey serum (TBS-plus).
Pretreatment for BrdU Immunohistochemistry.
For BrdU immunohistochemistry DNA has to be denatured. Sections were incubated in 50% formamide/50% 2× SSC buffer (0.3 M NaCl/0.03 M sodium citrate) at 65°C for 2 h, rinsed in 2× SSC, incubated in 2 M HCl for 30 min at 37°C, and rinsed in 0.1 M borate buffer pH 8.5 for 10 min.
Immunohistochemistry.
Free-floating sections were treated with 0.6% H2O2 to block endogenous tissue peroxidases. After pretreatment (see above), sections were kept in TBS-plus for 1 h and incubated in primary antibody against BrdU (monoclonal from mouse; Boehringer Mannheim, 1:400) for 12 h at 4°C. Sections were subjected to a biotinylated horse anti-mouse IgG secondary antibody (Jackson ImmunoResearch; 6 μl/ml) for 1 h. ABC reagent (Vectastain Elite, Vector Laboratories; 9 μl/ml) was applied for 1 h. Diaminobenzedine (Sigma; 0.25 mg/ml in TBS with 0.01% H2O2 and 0.04% nickel chloride) was used as chromogen. Differential interference contrast (DIC) photographs were obtained on an Olympus microscope. Slides were scanned with a Nikon Coolscan LS-1000 and the digital images processed as described below.
Immunofluorescence.
After pretreatment (see above) and blocking with TBS-plus, sections were incubated in an antibody mixture with antibodies against BrdU (monoclonal from rat; Accurate Scientific, Westbury, NY; 1:400), glial fibrillary acidic protein (GFAP) (polyclonal from guinea pig; Advanced Immunochemical, Long Beach, CA; 1:250) and calbindin D28K (polyclonal from rabbit; Swant, Bellinzona, Switzerland; 1:1000) for 12 h at 4°C. Secondary antibodies from donkey (fluorescein isothiocyanate to detect BrdU, CY5 for GFAP, and Texas Red for calbindin D28K; all from Jackson ImmunoResearch; 6 μl/ml) were applied for 1 h. Sections were coverslipped in polyvinyl alcohol with diazabicyclo-octane as antifading agent.
Fluorescent signals were detected with a confocal microscope (Zeiss/Bio-Rad MRC 1000) and the images were processed with Adobe photoshop 4.0. Only general adjustments were carried out and images were not otherwise digitally manipulated.
Stereology.
The total number of BrdU-positive cells in the subgranular layer was determined in coronal 40-μm sections, 240 μm apart, covering the complete left dentate gyrus in its rostro-caudal extension. BrdU-positive cells were counted within and one cell wide below the granule cell layer, ignoring cells in the uppermost focal plane and focusing through the thickness of the section (optical disector principle; for reviews, see refs. 14–16) to avoid oversampling errors. Data were statistically analyzed with ANOVA and Fisher post hoc test (statview 4.01 for Macintosh). A significance level of 5% was assumed. To allow further interpretation of the multiple comparisons of this study, Table 1 lists all P and F values.
Table 1.
Statistical analysis of strain comparisons: levels of significance
| C57BL/6 vs. BALB/c | C57BL/6 vs. CD1 | C57BL/6 vs. 129/SvJ | BALB/c vs. CD1 | BALB/c vs. 129/SvJ | CD1 vs. 129/SvJ | |
|---|---|---|---|---|---|---|
| Proliferation: 1 day after BrdU (see Fig. 2A); F, 3.311; P, 0.0470 | 0.0162 | 0.0164 | 0.0381 | NS | NS | NS |
| Survival: 4 weeks after BrdU (see Fig. 2A); F, 7.696; P, 0.0021 | NS | NS | 0.0158 | 0.0114 | NS (0.0789) | 0.0002 |
| Neuronal differentiation: 4 weeks after BrdU (see Fig. 2B); F, 0.392; P, 0.7609 | NS | NS | NS | NS | NS | NS |
| Glial differentiation: 4 weeks after BrdU (Fig. 2B); F, 2.767; P, 0.0781 | NS | NS | (0.0440) | NS | (0.0332) | (0.0288) |
| Volume of the granule cell layer (Table 2): F, 8.570; P, 0.0013 | NS | 0.0003 | NS | 0.0007 | NS | 0.0047 |
| Total granule cell number (Table 2): F, 16.664; P, <0.0001 | NS | <0.0001 | 0.0344 | <0.0001 | NS (0.0526) | 0.0012 |
| Neuronal density (Table 2): F, 0.815; P, 0.5042 | NS | NS | NS | NS | NS | NS |
| BrdU-positive cells as % of total granule cell number (Results): F, 3.386; P, 0.0441 | NS | NS | 0.0083 | NS | NS (0.0521) | 0.0323 |
Statistical analyses of strain comparisons were performed with factorial ANOVA and Fisher post hoc test. P ≤ 0.05 was considered to represent statistical significance. P between 0.05 and 0.1 and P < 0.05 in cases where the overall P did not reveal significance are given in parentheses. Significant comparisons are in bold. Overall F and P from ANOVA are listed in the first column (n is 5 in all groups). NS, not significant.
The corresponding sample volumes, the total volume of the dentate gyrus, and the absolute number of granule cells were determined in a parallel series of sections stained with Hoechst 33342 (Molecular Probes; 50 μg/ml TBS containing 0.1% Triton X-100 for 15 min), which binds DNA and labels nuclei with high contrast. For volumetric measurements with the Cavalieri estimation (15, 17) and determination of the absolute granule cell number, a semiautomatic stereology system, Stereoinvestigator 1.0 (MicroBrightfield), was used. A ×60 S-Plan-Apo oil objective (NA 1.40) was used on an Olympus microscope, equipped with a video camera, and all cell countings were done from the monitor. A 200-μm grid was superimposed over each section and granule cells in fields within the granule cell layer were counted in 15 × 15 × 40-μm sample volumes, disregarding cells that were in sharp focus in the uppermost focal plane (optical disector principle). The section thickness of 40 μm (microtome setting) was used in the disector because it was assumed that in an anatomical structure as homogeneous and as densely packed as the granule cell layer, counting errors at the exclusion plane (top of the section) would equal out with errors at the inclusion plane (bottom of the section). The net error by using the whole section thickness for the sample volume was considererd to be smaller than the error introduced by measuring the postprocessing section thickness on each slide and counting in a fixed fraction of it. The resulting neuronal density was multiplied by the total volume to estimate the total number of granule cells.
RESULTS
Proliferation.
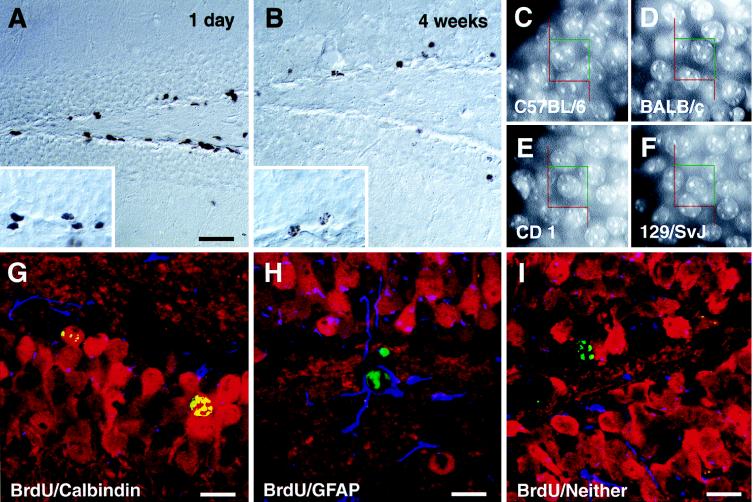
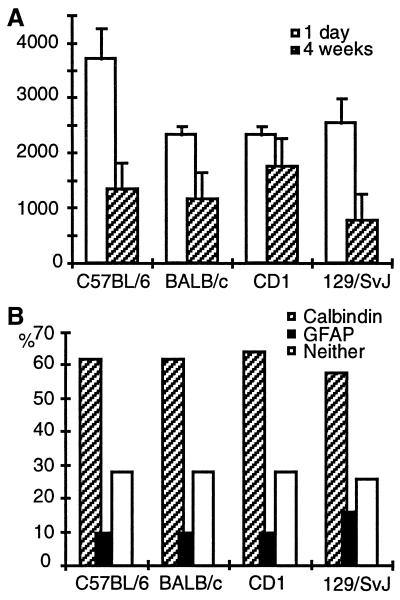
In the subgranular zone of the hippocampus, proliferating cells could be detected in all mouse strains (Fig. 1A). In addition, BrdU-positive cells were present in the hilar area (CA4) and in the molecular layer. Cell genesis, though not neurogenesis, in the hilus has been described (4, 5, 11). Density of BrdU-positive cells in the molecular layer did not exceed the very low general proliferative activity that could be found elsewhere in the brain. Quantification revealed that proliferative activity in C57BL/6 mice differed significantly from the other strains and was ≈1.5-fold higher (Fig. 2A and Table 1).
Figure 1.
Proliferation (A) and survival (B) of cells in the subgranular zone of C57BL/6 mice. BrdU-labeled cells 1 day after the last injection of BrdU (A) have dark irregular shaped nuclei (see Inset). Four weeks later (B) the number of BrdU-positive cells has decreased and the remaining cells have more rounded nuclei, sometimes with the typical chromatin structure of granule cells (see also insert and compare with C–F). Absolute granule cell numbers were determined stereologically using a 15 × 15 μm counting frame superimposed on a video image of Hoechst-33342-stained sections (C–F). No differences in the appearance of the granule cells can be noted, and the neuronal density in the granule cell layer is similar (see Table 2). Phenotypes of surviving newborn cells 4 weeks after the last injection were examined by means of immunofluorescence and confocal microscopy. Cells were categorized as to whether they showed double-labeling for BrdU (green) and granule cell marker calbindin (red) (G), BrdU and astrocytic marker GFAP (blue) (H), or for BrdU and neither of the two other markers (I). Quantification of phenotype distribution is found in Fig. 2B. (Bar in A = 75 μm for A and B and 25 μm for the Insets in A and B.) Counting frames in C–F are 15 × 15 μm. (Bars in G–I = 15 μm.)
Figure 2.
Quantification of BrdU-positive cells (A) and phenotype distribution (B). Numbers of BrdU-positive cells were determined 1 day after the last injection to assess proliferative activity (open bars in A) and 4 weeks later to address survival of newborn cells (hatched bars in A). Numbers are totals per granule cell layer (means ± SE). At 4 weeks cells were also examined for the expression of phenotypic marker proteins (B): calbindin for granule cells and GFAP for astrocytes (see Fig. 1 G–I). A total of 50 BrdU-positive cells per animal were analyzed. Statistical analysis is given in Table 1.
Survival.
Four weeks after the last injection of BrdU, survival of newborn cells was estimated (Fig. 1B). Fig. 2A and Table 1 give quantitative data: 129/SvJ had on average approximately only half as many surviving new cells as the other mouse strains and were significantly different from CD1 and close to significantly different (P = 0.0864) from C57BL/6. The ratio of numbers at 4 weeks vs. 1 day after the last injection reflects this survival pattern: 129/SvJ had a 4-week survival rate of only 1 of 4, whereas C57BL/6 had a rate of ≈1 of 3, BALB/c of 1 of 2, and CD1 of about more than 1 of 1.3.
Differentiation.
Colocalization of BrdU immunoreactivity with an immunoreaction for granule cell marker calbindin and astrocytic marker GFAP was investigated to determine the phenotype of newborn cells 4 weeks after the last injection of BrdU. Figs. 1 G–I show confocal microscopic images of triple-labeled newborn cells. Neuronal (Fig. 1G) and glial (Fig. 1H) differentiation of newborn cells could be detected in all four strains. Roughly one-third of the BrdU-positive cells in the granule cell layer did not show double-labeling, indicating either undifferentiated progenitor cells or differentiation into a phenotype not investigated here. Glial cells were found almost invariably within the subgranular zone. A trend (overall P = 0.0781) in phenotype difference was seen in 129/SvJ mice, where more newborn glial cells were found than in all other strains (Table 1).
New Neurons in the Dentate Gyrus of Adult Mice.
All of the above values are based on stereological data on the volume of the dentate gyrus (Table 2). CD1 mice had a significantly larger (by volume) dentate gyrus than the other strains. The absolute numbers of granule cells showed significant differences between strains: CD1 mice had significantly more granule cells than the other three strains, and 129/SvJ had significantly fewer (except for the comparison with BALB/c, where P = 0.0526). The packing density of neurons in the granule cell layer was not different between the strains (Table 2, Fig. 1 C–F).
Table 2.
Stereological data from the dentate gyrus
| C57BL/6 | BALB/c | CD1 (ICR) | 129/SvJ | |
|---|---|---|---|---|
| Volume of the granule cell layer (μm3) | 2.63 × 108 | 2.71 × 108 | 3.57 × 108 | 2.90 × 108 |
| ± 0.16 × 108 | ± 0.34 × 108 | ± 0.27 × 108 | ± 0.45 × 108 | |
| Total number of granule cells | 2.39 × 105 | 2.42 × 105 | 3.51 × 105 | 2.80 × 105 |
| ± 0.25 × 105 | ± 0.30 × 105 | ± 0.27 × 105 | ± 0.32 × 105 | |
| Neuronal density (cells/sample vol.) | 8.14 ± 0.18 | 9.19 ± 0.77 | 8.86 ± 0.18 | 8.77 ± 0.21 |
Stereological data from the dentate gyrus: volume, total granule cell number and neuronal density. All numbers are mean ± SE (n = 5). Sample volume was 9,000 μm3. Statistical analyses are in Table 1.
The number of BrdU-positive cells at 4 weeks can be expressed as a percentage of the total number of existing granule cells. The numbers are (mean ± SE): 0.58 ± 0.29% in C57BL/6, 0.48 ± 0.07% in BALB/c, 0.51 ± 0.12% in CD1, and 0.27 ± 0.03% in 129/SvJ. 129/SvJ mice differed from the other mice by having a significantly lower ratio (only the comparison BALB/c and 129/SvJ is not significant, but P = 0.0521; Table 1).
By multiplying this ratio by the percentage of BrdU/calbindin-positive cells (Fig. 2B), the number of new neurons with reference to the total number of granule cells can be derived. Thus, in 6 days (the period of BrdU injections) C57BL/6 mice produced at least 0.36% of their granule cell population as new calbindin-positive granule cells, BALB/c 0.30%, and CD1 0.32%, but 129/SvJ mice produced only 0.16%.
DISCUSSION
Neurogenesis in the Hippocampus of Adult Mice.
We here examined proliferation, survival, and phenotypic differentiation of neural progenitor cells in four mouse strains. In all four strains we found adult hippocampal neurogenesis, and strain differences could be identified in proliferation, survival, and differentiation; in total cell counts; and in volumetric parameters of the dentate gyrus.
Proliferation.
Strain differences in the numbers of BrdU-positive cells 1 day after the last injection do not necessarily reflect analogous differences in the population size of proliferating progenitor cells because BrdU incorporation is also influenced by cell cycle kinetics. As BrdU labels DNA only during S phase of the mitotic cycle and bioavailability of BrdU is ≈2 hours (18), one injection per day might not label all dividing cells during this 24-h period, but this regimen cannot cause overestimations. Nevertheless, the resulting numbers of BrdU-positive cells also include the progeny of labeled cells that divide again after the application of BrdU has been discontinued. Although these cells still represent newborn cells, this affects any statements about the exact time at which they were born. The rationale of spreading the injections over 6 days was to minimize the quantitative impact of continued divisions in the absence of exogenous BrdU.
The duration of the cell cycle for C57BL/6 mice at postnatal day 20 has been estimated to be 16 h with an S phase of 8 h (19). Related studies have shown that cell cycle lengthens with development (for references, see ref. 20). This result implies that C57BL/6 mice either have faster cell cycles than the other strains, or relatively larger populations of proliferating cells, or a combination of both. The net result, independent of these considerations, is more newborn cells in C57BL/6 than in the other strains.
Survival.
Comparing the numbers of BrdU-positive cells between 1 day and 4 weeks after the last injection reveals a dramatic decrease which is greatest in 129/SvJ (75%) and lowest in CD1 (23%).
A confounding interpretation of these results would be that cells had divided at such a high rate that BrdU was diluted below the threshold of detection. This possibility has to be taken into account, although it cannot explain the complete effect.
Stem cells can give rise to two daughter stem cells or divide asymmetrically—i.e., they renew themselves and give rise to one cell that differentiates (21). A decrease in BrdU-positive cells due to high proliferative activity would imply that the great majority of these cells divide symmetrically, because BrdU injections were spread over 6 consecutive days and cells exiting the cell cycle, at least in earlier divisions, would be detectable as BrdU-positive cells. Symmetric divisions would yield an enormous number of undifferentiated cells in the subgranular zone, an effect that is not seen in our study. However, ongoing cell death could balance this effect.
During embryonic development apoptotic cell death of newborn cells seems to play an important role in regulating the final number of cells. The occurrence of apoptosis, well described for proliferative regions of the developing brain (22), remains unevaluated for adult neurogenesis, although several studies have provided arguments for this assumption (23–25). We agree with the hypothesis that cells that “disappear” during the 4-week survival period are eliminated by apoptotis. Possibly for technical reasons (22, 26), however, it has so far not been possible to demonstrate apoptosis of BrdU-positive cells in regions of adult neurogenesis.
Independent of this consideration, a certain number of surviving cells differentiates (see below), thus proving that asymmetric division must occur. If proliferation were underestimated in 129Sv/J mice because of a hypothesized shorter cell cycle and labeling diluted out due to further symmetric divisions, the net survival rate (i.e., cells that differentiated) would yield a ratio even below 1 of 4. Thus, the existence of strain differences rather than the magnitude of differences between strains should be emphasized.
Differentiation.
After 4 weeks BrdU-positive cells colabeled with neuronal or glial markers in all strains. In vitro and re-implantation experiments have indicated that hippocampus-derived progenitor cells are pluripotent (27–29) and that the adult rat hippocampus contains premordial neural stem cells in the sense of a strict definition (30). However, we cannot conclude that BrdU-positive cells in the subgranular zone (as seen in Fig. 1 A, B, and G) are “identical” to those progenitor cells that can be propagated in vitro. Accordingly, we cannot determine whether the differentiated (calbindin- or GFAP-positive) cells stem from one pluripotent progenitor cell or two different, lineage-determined precursor cells.
The result that 129/SvJ mice differed from all other strains in the number of newborn cells differentiating into glia suggests two interpretations: (i) in 129/SvJ mice, cell fate decision for multipotent progenitor cells could be more often inclined toward glial differentiation, or (ii) in 129/SvJ mice, glial precursor cells receive relatively more stimulation to differentiate.
Absolute Number of Granule Cells.
The total numbers of neurons in the dentate gyrus of the four mouse strains examined fall into the range that has been reported for numerous other strains, and our results confirm that there are considerably strain differences with respect to the total granule cell number (31–34). 129/SvJ mice did not have fewer granule cells than the other strains, but in adulthood they produced fewer new granule cells. It is not quite clear in how far adult hippocampal neurogenesis in mice continuously adds new neurons to the granule cell layer or is part of a neuronal turnover with replacement of old granule cells. Wimer et al. have examined mice until postnatal day 84 and found a gradual increase in granule cell number (32). For adult hippocampal neurogenesis in rats there are arguments that the new cells are adding to the existing granule cells (35), and an increase in the granule cell number over the first year of life has been reported (36).
Regulation of Adult Hippocampal Neurogenesis.
It is well documented that hormonal status and age influence adult neurogenesis (5, 6, 37, 38). In addition, growth factors like fibroblast growth factor 2 and epidermal growth factor, whose effects have been demonstrated in vitro (27, 29, 39–41) influence proliferation and differentiation after intraventricular infusion (7, 42).
Excitatory input to the granule cell layer limits adult neurogenesis in the dentate gyrus; glutamatergic deafferentiation and treatment with a glutamate receptor antagonist both caused an increase in hippocampal neurogenesis (6, 43, 44). Interestingly, 129/Sv mice have been shown to be more susceptible to excitotoxic cell death in the hippocampus than C57BL/6 and BALB/c (45). This result might suggest that strain differences exist on the level of sensitivity to effects of glutamate (agonists) and the regulation of cell death. However, the complexity of this question is further increased by the finding that pilocarpine-induced seizures in a rat model of limbic epilepsy induced neurogenesis in the dentate gyrus (46).
Recently, psychosocial stress has been shown to down-regulate adult hippocampal neurogenesis in the tree shrew (47). A rest period prior to the start of the experiment, the use of female animals (which do not show territorial behavior), and housing of three or four animals in one standard cage were used to avoid stressful conditions in the study presented here.
On the other hand, overall sensory stimulation of the animals by housing them in an enriched environment resulted in an increased survival rate of newborn neurons and consequently a greater total number of granule cells (11). Together, these data suggest that regulation of adult hippocampal neurogenesis has activity-dependent components. This assumption is backed by data from song birds, which demonstrated that chickadees show more hippocampal neurogenesis in seasons that require them to cover larger territories and recall the location of food storage sites (9).
The genetic background provides the regulatory baseline upon which other factors can act, and their action will again be subject to genetic differences.
Strain Differences.
The largest difference in hippocampal neurogenesis could be found between C57BL/6 and 129/SvJ mice, which unfortunately are two strains that are widely crossed to generate transgenic or knockout animals. The resulting genetic pattern could have confounding consequences if effects supposedly related to the target gene could be caused by flanking background genes (48–50).
In addition, the pattern of strain differences was not uniform for the various parameters investigated (Table 1). For example, in 129/SvJ mice, which have the lowest number of surviving newborn cells, the same percentage turned into neurons as in the other strains. CD1 mice had (by volume) larger hippocampi but did not show a proportional increase in neurogenesis. Generally, the total number of granule cells was not a good indicator of adult neurogenesis. 129/SvJ mice, which had an average total number of granule cells, produced by far the lowest number of new granule cells (only 0.16% of the total granule cell number in 6 days).
Although different inbred mouse strains reach sexual maturity at a slightly different age [the first successful mating in C57BL/6 mice has been reported to be on average at 6.8 weeks, in BALB/c at 8.0 weeks, and in 129/SvJ at 7.9 weeks (51); outbred strains like CD1 normally surpass inbred strains], these differences are relatively minor and with the beginning of the experiment at an age of 9 weeks (8 weeks for 129/SvJ) all animals were likely to be sexually mature and “adult.” Chronologically, 129Sv/J mice in our study were 1 week younger than the other strains. This finding is unlikely to confound our results, as neurogenesis decreases with age (5, 52). Neurogenesis in 129/SvJ mice at the age of 9 weeks would likely be even lower than at 8 weeks.
Genetic and environmental control of neuronal cell numbers have also been investigated in the retina of mice from various strains, where it was found that about 75% of the measured variability in the number of retinal ganglion cells was heritable (53).
Although our results indicate that the genetic background strongly influences adult hippocampal neurogenesis, this does not imply that the genes in question are only those directly involved in the regulation of neurogenesis. A great number of genes that underlie system functions with known effects on neurogenesis (e.g., hormonal status) might indirectly be responsible for the strain differences found.
The multitude of factors involved might also explain possible species differences. To date most data on adult hippocampal neurogenesis have been derived from rats (1, 2, 4, 5) or mice (11, 52), but recently neurogenesis has been reported in the dentate gyrus of the adult tree shrew, considered to be phylogenetically between insectivores and primates (47). No hippocampal neurogenesis in adult rhesus monkeys has been found, although proliferating progenitor cells are present in the subgranular zone (54, 55). The question remains open whether under special conditions neurogenesis could be stimulated from these cells.
CONCLUSIONS
In future experiments, strain differences might be used to examine the genetic regulation of adult neurogenesis by means of classical genetics. At the same time, strain differences will influence design and interpretation of experiments using knockout or transgenic technologies.
Except for strain 129/SvJ, which produced half the average rate, the mice in our study produced on average more than 0.3% of their total granule cell number as new neurons over a period of 6 days; that is one new neuron per 2,000 existing granule cells every day. This rate is surprisingly high, and adult hippocampal neurogenesis therefore is not a negligible phenomenon. Future studies will have to show which functional role the new neurons play and whether the rate of adult hippocampal neurogenesis correlates with measures of hippocampal function, particularly learning and memory.
Acknowledgments
We thank M. L. Gage, L. Fisher and Th. Palmer for comments on the manuscript. This work was funded by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. G.K. was supported by Deutsche Forschungsgemeinschaft and H.G.K. by the Hereditary Disease Foundation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: BrdU, 5-bromodeoxyuridine; GFAP, glial fibrillary acidic protein.
To whom reprint requests should be addressed. e-mail: fgage@salk.edu.
References
- 1.Altman J, Das G D. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan M S, Hinds J W. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 3.Stanfield B B, Trice J E. Exp Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- 4.Cameron H A, Woolley C S, McEwen B S, Gould E. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen B S. Cell Mol Neurobiol. 1996;16:103–116. doi: 10.1007/BF02088170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig C G, Tropepe V, Morshead C M, Reynolds B A, Weiss S, van der Kooy D. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman S A, Nottebohm F. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnea A, Nottebohm F. Proc Natl Acad Sci USA. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnea A, Nottebohm F. Proc Natl Acad Sci USA. 1996;93:714–718. doi: 10.1073/pnas.93.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempermann G, Kuhn H G, Gage F H. Nature (London) 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 12.del Rio J A, Soriano E. Dev Brain Res. 1989;49:311–317. doi: 10.1016/0165-3806(89)90033-3. [DOI] [PubMed] [Google Scholar]
- 13.Saper C B. J Comp Neurol. 1996;364:5. doi: 10.1002/(SICI)1096-9861(19960101)364:1<5::AID-CNE1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Coggeshall R E, Lekan H A. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Gundersen H J, Bagger P, Bendtsen T F, Evans S M, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, Sørensen F B, Vesterby A, West M J. Acta Pathol Microbiol Immunol Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 16.West M J. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 17.Gundersen H J, Bendtsen T F, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, Sorensen F B, Vesterby A, West M J. Acta Pathol Microbiol Immunol Scand. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, Nowakowski R S, Caviness V S., Jr J Neurocytol. 1992;21:185–197. doi: 10.1007/BF01194977. [DOI] [PubMed] [Google Scholar]
- 19.Nowakowski R S, Lewin S B, Miller M W. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 20.Cai L, Hayes N L, Nowakowski R S. J Neurosci. 1997;17:2079–2087. doi: 10.1523/JNEUROSCI.17-06-02079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gage F H, Ray J, Fisher L J. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 22.Blaschke A J, Staley K, Chun J. Development (Cambridge, UK) 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- 23.Morshead C M, van der Kooy D. J Neurosci. 1992;12:249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron H A, Gould E. J Comp Neurol. 1996;369:56–63. doi: 10.1002/(SICI)1096-9861(19960520)369:1<56::AID-CNE4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.Sloviter R S, Dean E, Sollas A L, Goodman J H. J Comp Neurol. 1996;366:516–533. doi: 10.1002/(SICI)1096-9861(19960311)366:3<516::AID-CNE10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 26.Surh C D, Sprent J. Nature (London) 1994;372:100–103. [Google Scholar]
- 27.Gage F H, Coates P W, Palmer T D, Kuhn H G, Fisher L J, Suhonen J O, Peterson D A, Suhr S T, Ray J. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suhonen J O, Peterson D A, Ray J, Gage F H. Nature (London) 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 29.Palmer T D, Ray J, Gage F H. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 30.Palmer T D, Takahashi J, Gage F H. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 31.Wimer C C, Wimer R E. Dev Brain Res. 1989;48:167–176. doi: 10.1016/0165-3806(89)90073-4. [DOI] [PubMed] [Google Scholar]
- 32.Wimer R E, Wimer C C, Alameddine L. Brain Res. 1988;470:191–197. doi: 10.1016/0165-3806(88)90237-4. [DOI] [PubMed] [Google Scholar]
- 33.Wimer R E, Wimer C C, Vaughn J E, Barber R P, Balvanz B A, Chernow C R. Brain Res. 1978;157:105–122. doi: 10.1016/0006-8993(78)90999-x. [DOI] [PubMed] [Google Scholar]
- 34.West M J, Andersen A H. Brain Res Rev. 1980;2:317–348. doi: 10.1016/0165-0173(80)90012-0. [DOI] [PubMed] [Google Scholar]
- 35.Crespo D, Stanfield B B, Cowan W M. Exp Brain Res. 1986;62:541–548. doi: 10.1007/BF00236032. [DOI] [PubMed] [Google Scholar]
- 36.Bayer S A, Yackel J W, Puri P S. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 37.Cameron H A, Gould E. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 38.Gould E, Cameron H A, Daniels D C, Woolley C S, McEwen B S. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards L J, Kilpatrick T J, Bartlett P F. Proc Natl Acad Sci USA. 1992;89:8591–5859. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds B A, Tetzlaff W, Weiss S. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds B A, Weiss S. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 42.Kuhn H G, Winkler J, Kempermann G, Thal L J, Gage F H. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould E, Cameron H A, McEwen B S. J Comp Neurol. 1994;340:551–565. doi: 10.1002/cne.903400408. [DOI] [PubMed] [Google Scholar]
- 44.Gould E. Ann NY Acad Sci. 1994;743:73–92. doi: 10.1111/j.1749-6632.1994.tb55788.x. (discussion 92–93). [DOI] [PubMed] [Google Scholar]
- 45.Schauwecker P E, Steward O. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parent J M, Yu T W, Leibowitz R T, Geschwind D H, Sloviter R S, Lowenstein D H. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gould E, McEwen B S, Tanapat P, Galea L A M, Fuchs E. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. ; 188–189. [DOI] [PubMed] [Google Scholar]
- 49.Crusio W E. Trends Neurosci. 1996;19:186–187. doi: 10.1016/s0166-2236(96)20023-2. (discussion 188–189). [DOI] [PubMed] [Google Scholar]
- 50.Lathe R. Trends Neurosci. 1996;19:183–186. doi: 10.1016/s0166-2236(96)20022-0. (discussion 188–189). [DOI] [PubMed] [Google Scholar]
- 51.Silver L M. Mouse Genetics. New York: Oxford Univ. Press; 1995. [Google Scholar]
- 52.Reznikov K Y. Adv Anat Embryol Cell Biol. 1991;122:1–83. doi: 10.1007/978-3-642-76447-9. [DOI] [PubMed] [Google Scholar]
- 53.Williams R W, Strom R C, Rice D S, Goldowitz D. J Neurosci. 1996;16:7193–7205. doi: 10.1523/JNEUROSCI.16-22-07193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rakic P. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 55.Eckenhoff M F, Rakic P. J Neurosci. 1988;8:2729–2747. doi: 10.1523/JNEUROSCI.08-08-02729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]