Abstract
Telomerase is a specialized reverse transcriptase consisting of both RNA and protein components. Previous characterization of yeast telomerase function in vivo identified four EST (for ever shorter telomeres) genes that, when mutated, result in the phenotypes expected for a defect in telomerase. Consistent with this genetic prediction, the EST2 gene has recently been shown to encode the catalytic component of telomerase. Using an in vitro assay, we show here that telomerase activity is present in extracts prepared from yeast strains carrying est1-Δ, est3-Δ, and cdc13–2est mutations. Therefore, while these three genes are necessary for telomerase function in vivo, they do not encode components essential for core catalytic activity. When Est2p, the one EST gene product found to be essential for catalytic activity, was immunoprecipitated from extracts, the telomerase RNA subunit was also specifically precipitated, supporting the conclusion that these two components are in a stable complex.
Keywords: reverse transcriptase, DNA replication, telomerase RNA, TLC1, CDC13
In most eukaryotes, the complete replication of chromosomal termini requires the enzyme telomerase, which is a specialized reverse transcriptase. Using a portion of its RNA component as a template, telomerase adds simple G-rich repeat sequences onto chromosome ends (1). Genes encoding telomerase RNA subunits have been cloned from many organisms, including ciliated protozoa (2–5), budding yeast (6, 7), and mammals (8, 9). In contrast, identification and functional analysis of protein components of this enzyme are still at an early stage.
One approach toward the characterization of telomerase protein subunits employs biochemical purification of the telomerase ribonucleoprotein (RNP). This method has been most effective with ciliated protozoans such as Tetrahymena and Euplotes. These organisms maintain 40,000 to 40,000,000 individual DNA molecules in their transcriptionally active macronuclei, each capped with telomeres (10); as a consequence, they provide a relatively abundant source of the telomerase enzyme. Purification of the fully active Euplotes aediculatus telomerase has led to the identification of three subunits apparently present in equal stoichiometry: the 66-kDa RNA subunit and two proteins, p123 and p43 (11). Another biochemical approach identified two proteins, p95 and p80, as specifically associated with the Tetrahymena telomerase RNA and sedimenting with enzyme activity (12). Although no similarity has been observed between the Euplotes and Tetrahymena telomerase proteins, the identification of mammalian homologs of the Tetrahymena p80 protein that are associated with telomerase in mice, rats, and humans (13, 14) argues for the importance of this protein in telomere maintenance.
An alternative strategy to understand telomerase composition and function is a genetic one, using an organism amenable to in vivo analysis. This has the potential to uncover not only components of the core enzyme but also regulatory factors that may not have a tight association with telomerase and thus would not be identified by conventional purification. Applying this genetic approach in the yeast Saccharomyces cerevisiae led to the identification of four EST (ever shorter telomeres) genes (15, 16) that, when mutated, display the phenotypes expected for a telomerase-deficient strain. Epistasis analysis indicated that these four genes function in the same pathway for telomere replication as defined by the yeast telomerase RNA gene, TLC1 (6), and therefore have the potential to encode either components of the enzyme or requisite in vivo regulators (16–18).
These biochemical and genetic approaches converged with the recent demonstration that the p123 subunit of the Euplotes enzyme and the Est2 protein of S. cerevisiae are homologs (19). Most telling was the observation that both proteins have a set of amino acid sequence motifs previously shown to constitute a tertiary fold encompassing the active site of conventional reverse transcriptases (20–22). Single amino acid changes in highly conserved residues in these motifs in Est2p abolished yeast telomerase activity in vitro and conferred an est phenotype in vivo (19, 23), demonstrating that the yeast Est2 protein (Est2p) and Euplotes p123 are the catalytic subunits of their respective enzymes. The identification of p123/Est2p homologs in Schizosaccharomyces pombe and in humans suggests that this telomerase reverse transcriptase (TRT) subunit may be universal among telomerases (24).
In contrast, the functions of the remaining EST genes in telomere replication are still unfolding. Both Est1p and Cdc13p (identified as EST4 in the screen for est mutants; ref. 16) have the properties of single-strand telomere DNA-binding proteins (17, 18, 25), although genetic analysis argues for different roles for these two proteins in vivo. Est1p has been proposed to function, by means of its telomere DNA-binding activity, to direct telomerase to the chromosomal terminus (although the current data do not distinguish between Est1 serving this role as a component of telomeric chromatin or as a subunit of telomerase; ref. 17). In contrast, the Cdc13 protein has been proposed to play a dual role while bound to the telomere (18): Cdc13p protects the end of the chromosome (26), and second, Cdc13p regulates telomerase by mediating, either directly or indirectly, access of this enzyme to the terminus (18). Finally, EST3 encodes a novel 20-kDa protein with no homologs in the databases and no protein motifs that reveal its in vivo function at the telomere (D. K. Morris and V.L., unpublished results).
Although EST1, EST3, and EST4/CDC13 have critical roles in telomerase function in vivo, whether the products of these three genes are also essential for telomerase enzymatic activity per se has been unclear. Two previous reports, using two different in vitro assays for telomerase activity, reached opposite conclusions as to whether Est1p is required (27, 28), and the cdc13–2est mutant strain was tested (18) using an assay that was apparently not monitoring telomerase activity (subsequent studies suggested that the fraction used may contain a second polymerase activity; N. Lue, personal communication). Here we show that in an EST2- and TLC1-dependent enzyme assay (19), extracts prepared from est1-Δ, est3-Δ, or cdc13–2est strains retain telomerase activity. Thus, these three genes do not encode components required for core enzymatic activity.
MATERIALS AND METHODS
Yeast Strains.
The diploid strain DVL176 (Mata/Matα est1-Δ1::HIS3/EST1 est2-Δ1::URA3/EST2 est3-Δ1::LYS2/EST3 cdc13–2est/CDC13 ura3–52/ura3–52 lys2–801/lys2–801 ade2–101/ade2–101 trp1-Δ1/trp1-Δ1 his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1, CF-SUP11-TRP1) was derived by mating two isogenic haploid strains (Mata est1-Δ1 cdc13–2est and Matα est2-Δ1 est3-Δ1). Haploid derivatives for the experiment in Fig. 1 were generated by sporulation and dissection of DVL176. The est1-Δ, est2-Δ, and est3-Δ deletion mutations were detected by the nutritional marker linked to each mutation, whereas the cdc13–2est allele was detected by PCR amplification of the CDC13 gene and subsequent EcoRI digestion (the cdc13–2est allele results in the loss of an EcoRI site at amino acid 252 of Cdc13p; ref. 18). The haploid strain AVL78 (Mata leu2 trp1 ura3–52 prb prc pep4–1) was the parental strain for all experiments in Figs. 2, 3, 4. Null mutations in EST1 (est1-Δ3::LEU2; pVL415), EST2 (est2-Δ1::URA3; pVL363) and EST3 (est3-Δ2::URA3; pVL418) were introduced into AVL78 by one-step gene disruption; these three deletions removed 82%, 76%, and 63%, respectively, of coding sequence of the three EST genes. The cdc13–2est allele was introduced into AVL78 by a pop-in/pop-out strategy; the presence of the cdc13–2est allele in pop-out derivatives was detected as described above. Mutant transformants of AVL78 were screened for short telomeres and a senescence phenotype; in each case, extracts for telomerase assays were prepared from est mutant strains grown for as few generations as possible. The epitope-tagged derivative of Est2p (referred to as HA3-tagged Est2 in Fig. 4) was constructed by introducing three copies of the hemagglutinin nine-amino acid epitope (29) in frame at amino acid 11 of Est2p; this tagged version of the EST2 gene was present on a high copy (2 μ) plasmid and expressed by the ADH promoter (pVL782). When the epitope-tagged derivative was integrated into the genome by replacement of the wild-type EST2 gene, such that it was expressed under the control of the native EST2 promoter and in single copy, HA3-tagged Est2 was fully functional as assessed by telomere length and growth (T.R.H., data not shown).
Figure 1.
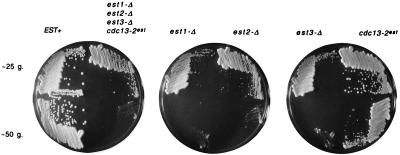
A quadruple-mutant est1-Δ est2-Δ est3-Δ cdc13–2est strain has the same phenotype as single-mutant est strains. Haploid quadruple- and single-mutant strains were generated by dissection of DVL176, and freshly germinated haploid spores of the indicated genotype were streaked for single colonies on YEPD (yeast extract/peptone/dextrose) rich medium plates. Two successive streak-outs, differing from each other by ≈25 generations (g.), were assembled on YEPD plates and incubated for 3 days at 30°C prior to photography. A total of four to five strains for each genotype were analyzed in parallel, with similar results observed for each genotype; a representative example is shown for each.
Figure 2.
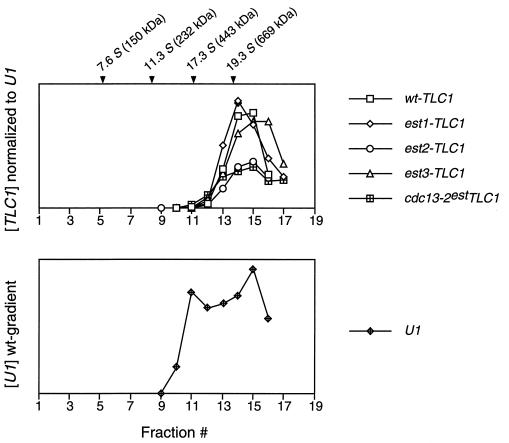
Sedimentation of the yeast telomerase RNP from est mutant strains. Yeast extracts were fractionated by glycerol gradient sedimentation, and the yeast TLC1 RNA (Upper) and U1 snRNA (Lower, shown for the EST+ strain) were quantitated by PhosphorImager analysis following Northern blotting. The U1 snRNA distribution was consistently bimodal, which may represent the free snRNP and higher-order complexes. Marker proteins were run in a parallel gradient (alcohol dehydrogenase, 7.6S; catalase, 11.3S; apoferritin, 17.3S; and thyroglobulin, 19.3S).
Figure 3.
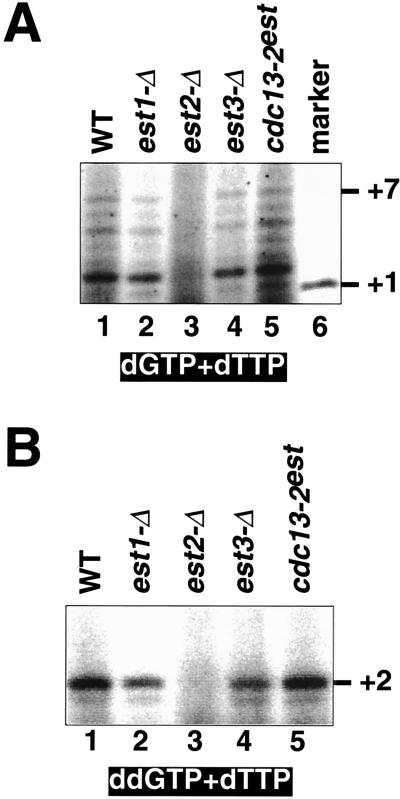
Telomerase activity is present in est1-Δ, est3-Δ, and cdc13–2est strains. (A) Telomerase activity as measured in the standard reaction, in the presence of [α-32P]dTTP and dGTP. Pooled glycerol gradient fractions were concentrated, to equalize the amount of telomerase RNA in the various samples. The +1 marker was generated by terminal deoxynucleotidyltransferase extension of the same primer used in the telomerase assays with [α-33P]ddTTP. (B) Enzyme activity monitored in the presence of [α-32P]dTTP and the chain-terminator ddGTP, using the same pooled fractions as in A.
Figure 4.
Est2p is in a complex with the yeast telomerase RNA. Immunoprecipitation of Est2p and TLC1 RNA from extracts of a HA3-tagged Est2 strain and of a control untagged Est2 strain by incubation with the anti-HA antibody. (A) Western blot, using 12CA5 antibody; lanes 1 and 2, 5 μl (35 μg) of extracts from strains carrying pVL782 (HA3-tagged Est2) or pVL369 (untagged Est2), respectively; lanes 3 and 4, immunoprecipitates derived from 550 μl of the same extracts used in lanes 1 and 2, respectively. (B) Western blot as in A; lanes 1 and 2, 10 μl (60 μg) of extracts from strains carrying pVL782 (HA3-tagged Est2) or pVL406 (untagged Est2), respectively; lanes 3 and 4, immunoprecipitates derived from 250 μl of the same extracts used in lanes 1 and 2, respectively. A longer exposure is shown for lanes 1 and 2 than for lanes 3 and 4; the ≈50-kDa band in lanes 1 and 2 is a protein that crossreacts with the 12CA5 antibody, detected on long exposures. (C) Northern blot, using TLC1 and U1 probes; lane 1, molecular mass markers; lanes 2 and 3, RNA extracted from the same extracts and extract volume as in lanes 1 and 2, part A; lanes 4 and 5, RNA extracted from the same immunoprecipitates (and same volume) as shown in lanes 3 and 4, part A. (D) Northern blot, using TLC1 and U1 probes; lanes 1 and 2, RNA extracted from the same extracts and extract volume as in lanes 1 and 2, part B; lanes 3 and 4, RNA extracted from the same immunoprecipitates (and same volume) as shown in lanes 3 and 4, part B.
Telomerase Assays.
Two liters of wild-type or est mutant cultures were grown and extracts were prepared as described previously (19). After concentration and glycerol gradient centrifugation, ≈20 fractions were collected and assayed for the presence of the telomerase TLC1 RNA and the U1 small nuclear RNA (snRNA). The amount of TLC1 RNA in each fraction was quantitated by PhosphorImager (Molecular Dynamics) analysis of Northern blots, and TLC1-containing fractions were pooled and concentrated 3- to 12-fold (to 50 μl); to compare different strains, the amount of pooled fraction subject to concentration was adjusted such that all enzyme assays contained an identical quantity of the telomerase RNA. Telomerase assays were performed as previously described (19): enzyme assays [typically 1 μl of concentrated pooled fractions in 10 μl of 20 mM Tris-acetate, pH 7.5/1 mM MgCl2/30 mM potassium glutamate/1 mM dithiothreitol/1 μM telomeric primer/100 μM dGTP or ddGTP and 1.5 μl of [α-32P]dTTP (800 Ci/mmol; 1 Ci = 37 GBq)] were incubated for 1 hr at 30°C. The telomeric primer was 5′-TGTGGTGTGTGTGGG-3′.
Immunoprecipitations.
AVL78 transformed with pVL782 (HA3-tagged Est2), pVL369 or pVL406 (untagged versions of Est2) were grown in 500 ml of selective media to OD600 = 1.0. Extracts were prepared by lysis with glass beads (0.5 mm, Biospec) five times for 1 min at maximum speed in a bead-beater (Biospec) in TMG buffer [10 mM Tris⋅HCl, pH 8.0/1 mM MgCl2/10% (vol/vol) glycerol] with 200 mM NaCl and cleared twice by centrifugation for 10 min at 14,000 rpm in a microcentrifuge at 4°C to yield ≈2 ml of extract at 5–7 mg/ml. Extract (1.65 ml in Fig. 4 A and C or 0.75 ml in Fig. 4 B and D) was incubated with 1 μl of the anti-HA antibody (12CA5 antibody; Babco, Richmond, CA) and rotated gently at 4°C for 1 hr; 20 μl of protein A/G-agarose beads (Oncogene Science) was added, and rotation was continued for 2 more hours. Beads were collected by pulsing in a microcentrifuge (≈0.25 sec, <1,000 rpm), and washed four times for 5–10 min each; three washes were in TMG, 200 mM NaCl, and 0.5% Tween-20, and the last wash was in TMG. The final agarose bead pellet (20 μl) was resuspended in 40 μl of TMG. For Western blotting, cleared crude extracts or immunoprecipitate samples were resolved by 0.1% SDS/8% PAGE, transferred to nitrocellulose, probed with 12CA5 antibody, and visualized by using horseradish peroxidase-conjugated goat anti-mouse antibody and ECL (enhanced chemiluminescence; Amersham).
Northern Blot Hybridization.
TLC1 and U1 RNAs were prepared and detected by Northern blotting on 7 M urea/4% PAGE. Blots were hybridized with random-primed full-length TLC1 and end-labeled antisense U1 oligonucleotide (5′-CTACTATTGGAAGCGCATGTTTG-3′), and efficiency of immunoprecipitation was determined by quantitation by PhosphorImager analysis. Size markers were generated by end-labeling HaeIII-digested φX174 DNA, followed by denaturation.
RESULTS
EST1, EST3, and EST4/CDC13 Function in the Same in Vivo Pathway as Telomerase.
Prior genetic characterization had shown that yeast strains carrying a tlc1 null mutation and a second mutation in one of the four EST genes do not show any enhancement of phenotype, relative to either single mutant strain; similar results were obtained when double-mutant combinations with an est1 null mutation were examined (16). This indicated that all five genes operate in the same genetically defined pathway. To test further the conclusions from this epistasis analysis, we constructed a haploid strain containing all four est mutations (est1-Δ est2-Δ est3-Δ cdc13–2est) and compared the senescence phenotype of this quadruple-mutant strain with that of each single-mutant strain. Fig. 1 shows the growth characteristics for these five mutant strains at two different time points, separated by ≈25 generations, in parallel with a wild-type EST+ strain. The senescence of the quadruple-mutant strain was indistinguishable from that displayed by the est1-Δ, est2-Δ, and est3-Δ strains (the cdc13–2est strain exhibited a slightly delayed senescence phenotype relative to the other mutant strains, consistent with previous results for this strain; refs. 16 and 18). This observation supports the hypothesis that the four EST genes are required for a single telomerase-mediated process for telomere replication.
For all five haploid strains (both the quadruple-mutant and each single-mutant strain), a marked senescence phenotype manifested itself after only 25 to 50 generations of growth after sporulation and dissection of the heterozygous diploid parent strain (Fig. 1). This is in sharp contrast to the longer phenotypic delay (75 to 100 generations) usually observed for est mutant strains (15, 16). A moderate enhancement of senescence had been previously observed for haploid strains derived from diploid parents heterozygous for two of the EST/TLC1 genes (16). This enhancement was proposed to be due to additive haplo-insufficiency of the genes in this pathway; e.g., in a diploid strain where one copy of an EST or TLC1 gene is deleted, the amount of Est protein or telomerase RNA produced would be slightly below that necessary for wild-type telomere function. Consistent with this proposal, multiply heterozygous diploids displayed slightly shortened telomeres, relative to a diploid strain with all of the EST and TLC1 genes present in two copies (D. K. Morris and V.L., unpublished data). Haploid strains produced from such a strain would be expected to have a slightly shorter starting telomere length and as a consequence, a more rapid appearance of the senescence phenotype. The haploid strains shown in Fig. 1, generated from the quadruple heterozygote, show a further exaggeration of phenotype relative to that observed with haploid strains derived from a double heterozygote (ref. 16 and V.L., data not shown), providing support for the idea that the observed haplo-insufficiency is additive. Although we do not understand the molecular basis for the additive effects, the reductions in gene dosage appear to be altering the levels of a complex or a set of interacting complexes.
Est1p, Est3p, and the Cdc13 Function Defined by the cdc13-2est Mutation Are Not Required for Yeast Telomerase Catalysis.
The genetic analysis of est1, est3, and cdc13–2est mutant strains indicated that these three genes were as critical in vivo for telomerase function as EST2, the latter encoding the catalytic core of the enzyme. To test whether these three gene products were also essential for in vitro enzyme activity, telomerase was assayed from extracts prepared from each mutant strain, in parallel with extracts from est2-Δ and EST+ strains. Extracts were fractionated by glycerol gradient centrifugation and fractions were monitored for the TLC1 RNA subunit by Northern blotting, along with U1 snRNA as a control. Yeast telomerase from a wild-type EST+ strain sediments as an ≈20S particle (ref. 19; Fig. 2), and the sedimentation profile was similar to that of wild type for all four est mutant strains (Fig. 2).
Telomerase activity was assayed by incubation of pooled fractions containing the telomerase RNA with a single-stranded telomeric oligonucleotide. In the presence of dGTP and dTTP, a wild-type yeast extract elongated the primer by up to 7 nucleotides (Fig. 3A), consistent with the alignment of substrate with the RNA template and a single round of extension, as previously observed for yeast telomerase (19, 23, 27, 30). The same product distribution and roughly comparable levels of activity were observed in est1-Δ, est3-Δ, and cdc13–2est yeast strains (Fig. 3A), but no activity was detectable in the est2-Δ strain, in agreement with our previous report (19). A second assay monitored extension of the same primer in the presence of dTTP and the chain-terminating dideoxynucleotide analog ddGTP (Fig. 3B). Under these conditions, the predicted alignment of the primer on the template should allow extension of the primer by only two nucleotides. Because this latter assay concentrates the reaction products in two bands, it also permits a greater differentiation between telomerase-plus and telomerase-minus yeast extracts. Again, activity was present in wild-type extracts as well as est1-Δ, est3-Δ, and cdc13–2est yeast but was completely absent from the est2-Δ strain (Fig. 3B).
As mentioned above, there is no substantial change in the sedimentation coefficient of telomerase in est1-Δ, est3-Δ, and cdc13–2est mutant strains, within the limits of resolution of these gradients (Fig. 2A). This could be used to argue that the 82-kDa Est1, 20-kDa Est3, and 105-kDa Cdc13 proteins are not integral components of the telomerase complex. However, this argument is inconsistent with the fact that removal of the 103-kDa Est2 protein, encoding an essential component of the enzyme (ref. 19 and see below), also does not substantially alter the sedimentation position of the RNA (Fig. 2A). It is possible that yeast telomerase is a multisubunit complex of sufficient size that the absence of one subunit is not sufficient to perturb the sedimentation of the 20S complex. Another alternative is that when the telomerase RNP is disrupted, the 430-kDa yeast telomerase RNA (which, when deproteinized, sediments at ≈17 S; ref. 19) may form nonspecific associations with other RNA-binding proteins present in the extract.
Est2p Is in a Complex with the Telomerase RNA Subunit.
The Euplotes p123 subunit was purified as an integral component of the Euplotes RNP (11), and photocrosslinking studies have shown that the telomeric primer interacts with both a protein of approximately this size and the telomerase RNA (31), supporting a direct association between these two subunits. To test whether the Est2 protein is in a complex with the yeast telomerase RNA, Est2p immunoprecipitates were examined for preferential coprecipitation of the TLC1 RNA. Extracts prepared from yeast strains expressing high levels of HA3-tagged or untagged versions of Est2p were incubated sequentially with anti-HA antibody and protein A/G agarose, and immune complexes were collected by gentle centrifugation, washed, and subjected to Northern and Western blotting. Fig. 4 shows two different experiments comparing the amounts of TLC1 and U1 snRNA co-immunoprecipitated with tagged versus untagged versions of Est2p. In both cases, there was a substantial enrichment (≈20- to 50-fold) in the amount of TLC1 RNA precipitated with HA3-tagged Est2p, relative to the untagged control (compare lanes 4 and 5 in Fig. 4C and lanes 3 and 4 in Fig. 4D), whereas no enrichment in the U1 snRNA was detected. This finding demonstrates that there is a specific association between the yeast telomerase RNA and Est2p, and it also indicates that a significant portion of the cellular TLC1 population is Est2p-associated, as expected if both are components of the telomerase complex.
DISCUSSION
A previous genetic screen designed to identify genes required for telomere replication in yeast identified four EST genes (15, 16). Catalytic components of telomerase would be expected to be among the proteins encoded by the genes recovered in such a screen and, indeed, the Est2 protein contains the reverse transcriptase active site residues that catalyze telomere extension (19, 23). Other EST genes could in principle also contribute directly to core enzymatic activity or could provide essential nonenzymatic functions as described below. In this study, we applied an in vitro telomerase assay (19) to distinguish between enzymatic and nonenzymatic functions. Our principle finding is that the Est1 and Est3 proteins are dispensable and the Cdc13est mutant protein is sufficient for yeast telomerase catalytic activity in vitro, indicating that they are neither required components of the telomerase core enzyme nor telomerase assembly factors. This work confirms a previous report of EST1-independent telomerase activity (27) and further emphasizes that proteins in addition to telomerase are required for telomerase-mediated telomere maintenance in vivo.
There are several possibilities regarding the function(s) of these additional EST proteins. They could be components of telomeric chromatin that are requisite for telomerase access/recruitment, or telomerase subunits that function as specificity factors and mediate recognition of the chromosomal terminus. Alternatively, one or more of these could be telomerase-associated proteins that modulate enzyme activity, such as processivity or the stabilization or dissociation of primer/template interactions. Finally, they may not be stably associated with either telomerase or its telomere substrate, but may act transiently to accomplish telomerase localization or transport.
The biochemical properties of Est1p and Cdc13p suggest more detailed models for their in vivo function. Both proteins exhibit sequence-specific binding to single-stranded yeast telomeric oligonucleotides in vitro (17, 18, 25), indicating that they are each capable of binding the single-strand G-rich extension present at the chromosomal terminus. The Est1 protein co-immunoprecipitates with the yeast telomerase RNA (28, 32) and binds RNA in vitro (17). Est1p binding to telomeric DNA also shows a strong preference for a free single-stranded 3′ end (17). These data suggest that Est1p interacts with telomerase and may assist in bringing the primer terminus to the enzyme active site, although whether Est1p does so as an integral component of the enzyme or as a factor bound to the end of the chromosome has not yet been determined. In contrast, several lines of evidence suggest that Cdc13p is a component of telomeric chromatin that functions in telomerase regulation as well as protection of the chromosomal terminus (18, 25, 26). In particular, the identification of an est-like allele of CDC13 has led to the proposal that Cdc13p, while bound to the telomere, positively regulates telomerase access, with access eliminated by the cdc13–2est mutation (18). An important untested component of this model was that telomerase activity should be present in cdc13–2est extracts, despite the fact that this strain exhibits an in vivo phenotype virtually identical to that of a telomerase-defective yeast strain. This aspect of the model is now confirmed by the experiments presented in Fig. 3.
We have previously reported the identification of homologous genes encoding the catalytic subunits of the Euplotes and S. cerevisiae telomerase enzymes (19). We provide additional evidence here that Est2p is in a complex with the yeast telomerase RNA (see also ref. 19), consistent with the demonstration by Counter et al. (23) that telomerase activity can be immunoprecipitated with Est2p. This mimics the association previously shown for p123 and the Euplotes telomerase RNA (11), and shows that the association between the telomerase RNA and the telomerase catalytic subunit is conserved between distantly related species.
These results contribute to a growing body of evidence that telomere replication and maintenance by telomerase involve a number of factors that are required in vivo for modification or regulation of enzyme activity. Whereas the catalytic protein component of the core enzyme appears to be conserved across diverse eukaryotes (19, 24), other telomerase-associated proteins appear to have a restricted distribution among species. For example, there do not appear to be homologs of the Tetrahymena telomerase-associated p80 and p95 proteins in the completely sequenced yeast genome, despite the fact that mammalian homologs of p80 have been identified (13, 14). One possibility is that one or more of the Est proteins is a functional homolog of either p80 or p95. Resolution of these matters will require identification of specific roles for these additional telomerase-related proteins.
Acknowledgments
We thank Danna Morris for construction and characterization of strains used in Figs. 2 and 3, and Dan Gottschling, Art Lustig, and Toru Nakamura for comments on the manuscript. This work was supported in part by grants from the National Institutes of Health and Geron Corporation (V.L.). T.R.C. is an Investigator of the Howard Hughes Medical Institute and an American Cancer Society Professor.
ABBREVIATIONS
- RNP
ribonucleoprotein
- snRNA
small nuclear RNA
- HA
hemagglutinin
References
- 1.Blackburn E H, Greider C W, editors. Telomeres. Plainview, NY: Cold Spring Harbor Lab. Press; 1995. [Google Scholar]
- 2.Greider C W, Blackburn E H. Nature (London) 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 3.Shippen-Lentz D E, Blackburn E H. Science. 1990;247:546–552. doi: 10.1126/science.1689074. [DOI] [PubMed] [Google Scholar]
- 4.Romero D P, Blackburn E H. Cell. 1991;67:343–353. doi: 10.1016/0092-8674(91)90186-3. [DOI] [PubMed] [Google Scholar]
- 5.Lingner J, Hendrick L L, Cech T R. Genes Dev. 1994;8:1984–1998. doi: 10.1101/gad.8.16.1984. [DOI] [PubMed] [Google Scholar]
- 6.Singer M S, Gottschling D E. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 7.McEachern M J, Blackburn E H. Nature (London) 1995;376:403–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Funk W D, Wang S-S, Weinrich S L, Avilion A A, Chiu C-P, Adams R R, Chang E, Allsopp R C, Yu J, Le S, West M D, Harley C B, Andrews W H, Greider C W, Villeponteau B. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 9.Blasco M A, Funk W, Villeponteau B, Greider C W. Science. 1995;269:1267–1270. doi: 10.1126/science.7544492. [DOI] [PubMed] [Google Scholar]
- 10.Prescott D M. Microbiol Rev. 1994;58:233–267. doi: 10.1128/mr.58.2.233-267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingner J, Cech T R. Proc Natl Acad Sci USA. 1996;93:10712–10717. doi: 10.1073/pnas.93.20.10712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins K, Kobayashi R, Greider C W. Cell. 1995;81:677–686. doi: 10.1016/0092-8674(95)90529-4. [DOI] [PubMed] [Google Scholar]
- 13.Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass M B, Arruda I, Robinson M O Amgen EST Program. Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama J-I, Saito M, Nakamura H, Matsuura A, Ishikawa F. Cell. 1997;88:875–884. doi: 10.1016/s0092-8674(00)81933-9. [DOI] [PubMed] [Google Scholar]
- 15.Lundblad V, Szostak J W. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 16.Lendvay T S, Morris D K, Sah J, Balasubramanian B, Lundblad V. Genetics. 1996;144:1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virta-Pearlman V, Morris D K, Lundblad V. Genes Dev. 1996;10:3094–3104. doi: 10.1101/gad.10.24.3094. [DOI] [PubMed] [Google Scholar]
- 18.Nugent C, Hughes T R, Lue N F, Lundblad V. Science. 1996;274:249–252. doi: 10.1126/science.274.5285.249. [DOI] [PubMed] [Google Scholar]
- 19.Lingner J, Hughes T R, Shevchenko A, Mann M, Lundblad V, Cech T R. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 20.Kohlstaedt L A, Wang J, Friedman J M, Rice P A, Steitz T A. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 21.Jacobo-Molina A, Ding J, Nanni R G, Clark A D, Lu X, Tantillo C, Williams R L, Kamer G, Ferris A L, Clark P, Hizl A, Hughes S H, Arnold E. Proc Natl Acad SciUSA. 1993;90:6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong Y, Eickbush T H. EMBO J. 1990;9:3353–3362. doi: 10.1002/j.1460-2075.1990.tb07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Counter C M, Meyerson M, Eaton E N, Weinberg R A. Proc Natl Acad Sci USA. 1997;94:9202–9207. doi: 10.1073/pnas.94.17.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 25.Lin J-J, Zakian V A. Proc Natl Acad Sci USA. 1996;93:13760–13765. doi: 10.1073/pnas.93.24.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garvik B, Carson M, Hartwell L. Mol Cell Biol. 1995;15:6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohn M, Blackburn E H. Science. 1995;269:396–400. doi: 10.1126/science.7618104. [DOI] [PubMed] [Google Scholar]
- 28.Lin J-J, Zakian V A. Cell. 1995;81:1127–1135. doi: 10.1016/s0092-8674(05)80017-0. [DOI] [PubMed] [Google Scholar]
- 29.Wilson I A, Niman H L, Houghten R A, Cherenson A R, Connolly M L, Lerner R A. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 30.Prescott J, Blackburn E H. Genes Dev. 1997;11:528–540. doi: 10.1101/gad.11.4.528. [DOI] [PubMed] [Google Scholar]
- 31.Hammond P W, Lively T N, Cech T R. Mol Cell Biol. 1997;17:296–308. doi: 10.1128/mcb.17.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner B R, Hidaka K, Futcher B. Proc Natl Acad Sci USA. 1996;93:2817–2821. doi: 10.1073/pnas.93.7.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]