Abstract
The TTX-sensitive Nav1.7 (PN1) Na+ channel α subunit protein is expressed mainly in small dorsal root ganglion (DRG) neurones. This study examines immunocytochemically whether it is expressed exclusively or preferentially in nociceptive primary afferent DRG neurones, and determines the electrophysiological properties of neurones that express it. Intracellular somatic action potentials (APs) evoked by dorsal root stimulation were recorded in L6/S1 DRG neurones at 30 ± 2 °C in vivo in deeply anaesthetised young guinea-pigs. Each neurone was classified, from its dorsal root conduction velocity (CV) as a C-, Aδ- or Aα/β-fibre unit and from its response to mechanical and thermal stimuli, as a nociceptive, low threshold mechanoreceptive (LTM) or unresponsive unit. Fluorescent dye was injected into the soma and Nav1.7-like immunoreactivity (Nav1.7-LI) was examined on sections of dye-injected neurones. All C-, 90 % of Aδ- and 40 % of Aα/β-fibre units, including both nociceptive and LTM units, showed Nav1.7-LI. Positive units included 1/1 C-LTM, 6/6 C-nociceptive, 4/4 C-unresponsive (possible silent nociceptive) units, 5/6 Aδ-LTM (D hair), 13/14 Aδ-nociceptive, 2/9 Aα/β-nociceptive, 10/18 Aα/β-LTM cutaneous and 0/9 Aα/β-muscle spindle afferent units. Overall, a higher proportion of nociceptive than of LTM neurones was positive, and the median relative staining intensity was greater in nociceptive than LTM units. Nav1.7-LI intensity was clearly positively correlated with AP duration and (less strongly) negatively correlated with CV and soma size. Since nociceptive units tend overall to have longer duration APs, slower CVs and smaller somata, these correlations may be related to the generally greater expression of Nav1.7 in nociceptive units.
Voltage-gated Na+ channels are important for generation and conduction of action potentials (APs). They are composed of α subunits that form the voltage-sensitive and ion-selective pore, and β subunits that can modulate the properties of the α subunit (see Catterall, 2000). Na+ channel α subunits in dorsal root ganglia (DRGs) include tetrodotoxin-resistant (TTXR) and TTX-sensitive (TTXS) channel subunits. The TTXS α subunit Nav1.7 (PN1 or peripheral nerve type 1), present in DRG tissue (Sangameswaran et al. 1997; Toledo-Aral et al. 1997) is the rat homologue of both the human neuroendocrine Na+ channel (hNE) (Klugbauer et al. 1995) found in adrenal and thyroid glands, and the rabbit Na+ channel NaS (Belcher et al. 1995).
Nav1.7 protein is expressed in DRG and sympathetic ganglion neurones (Toledo-Aral et al. 1997). Nav1.7 mRNA is at higher levels in the peripheral than the central nervous system, with some studies finding no Nav1.7/ Nav1.7 mRNA in the rat CNS (Klugbauer et al. 1995; Toledo-Aral et al. 1997). Thus, while not exclusively located in DRG neurones, Nav1.7 is much more highly expressed in these than in CNS neurones. Despite the distribution of Nav1.7 mRNA in DRG neurones of all sizes (Black et al. 1996), anti-Nav1.7 antibodies show more intense labelling of small than large DRG neurones in adult (Porreca et al. 1999, Gould et al. 2000) but not in fetal rats (Toledo-Aral et al. 1997). Thus Nav1.7 protein, but not mRNA appears to be more highly expressed in small than large adult DRG neurones.
It is important to determine which Na+ channel subunits are restricted to, or preferentially expressed in, nociceptive neurones, since such subunits may prove to be useful targets for novel analgesics. Small DRG neurones are often assumed to be nociceptive. Interest has therefore been focussed on Na+ channel α subunits that are expressed preferentially in these neurones. These include the TTXR subunits Nav1.8 (SNS/PN3) (Akopian et al. 1996; Tzoumaka et al. 1997) and Nav1.9 (NaN/SNS2) (Dib-Hajj et al. 1998; Tate et al. 1998) and the TTXS subunit protein Nav1.7. However, since cell size alone is an unsafe predictor of nociceptive function (S. N. Lawson, unpublished observations, also see Hoheisel et al. 1994), direct examination of sensory properties is essential to establish whether Nav1.7 protein in DRGs is limited to, or preferentially expressed in, nociceptive neurones.
APs in small sized DRG neurones have Na+ inward currents with both TTXR and TTXS components. The TTXR inward current in the AP is thought to be via the Nav1.8 channel subunit (Akopian et al. 1996), and although Nav1.7 is thought to be involved in impulse initiation (Cummins et al. 1998), its contribution to fibre conduction velocity (CV) and to the inward current in somatic APs is not clear.
We have therefore examined in DRG neurones (a) whether detectable Nav1.7-LI is only in, or is more intense in, nociceptive neurones and (b) whether Nav1.7-LI levels in neuronal somata are related to active membrane properties of somata or fibres. To achieve this, we have made intracellular voltage recordings from individual DRG neurones in anaesthetised guinea-pigs in vivo, identified their sensory properties and passed fluorescent dye into their somata. We then determined immunocytochemically the Nav1.7-LI of these identified neurones.
An abstract referring to preliminary data included in this paper has been published (Djouhri et al. 2000).
Methods
All experimental procedures used conformed with the UK Animals (Scientific Procedures) Act 1986.
Guinea-pigs were prepared for electrophysiological recordings as previously described (Djouhri et al. 1998) and sensory properties of units were established as described in full in Lawson et al. (1997). Briefly the methods were as follows. Young female guinea-pigs (weight 160-300 g) were deeply anaesthetised with sodium pentobarbitone with an initial dose of 50 mg kg−1, i.p. A tracheotomy allowed artificial ventilation and end-tidal CO2 monitoring. Further anaesthetic in doses of 10 mg kg−1 as needed (about every hour) to maintain areflexia (tested regularly with pinch of the forepaw), was given via a cannula in the left carotid artery. In later experiments, blood pressure was monitored throughout the experiment.
The ilium and the L1 vertebra were clamped. A laminectomy exposed L6 and S1 DRGs. A small silver platform beneath the DRG increased stability during recording. Adipose tissue over the DRG was removed, taking care not to damage the DRG surface blood vessels. The dorsal root of the DRG under study was cut as close as possible to the spinal cord and a pair of bipolar platinum electrodes inserted under it. This was used for (a) establishing, with extracellular recording, the receptive field region of the whole DRG to natural stimulation and (b) stimulation of the dorsal root during intracellular recordings. Recordings were made in a paraffin pool maintained at 28.0-31.5 °C.
Intracellular recordings
Just before recording, a muscle relaxant was administered intra-arterially (i.a.) accompanied always by an additional dose (10 mg kg−1, i.a.) of the anaesthetic. The muscle relaxant used was either gallamine triethiodide (Flaxedil, 2 mg kg−1i.a.) or pancuronium (0.5 mg ml−1i.a.). These same doses of muscle relaxant and anaesthetic (always given together) were administered regularly (approximately hourly). The frequency and amount of anaesthetic administered during the neuromuscular blockade was the same as that required to maintain complete areflexia in the 2 h prior to administering the Flaxedil. Blood pressure recordings in later experiments of the series with this anaesthetic regime were very stable throughout the experiment, indicating deep anaesthesia.
Intracellular recordings from DRG somata were made with glass microelectrodes filled with a fluorescent dye. The fluorescent dyes were Lucifer yellow CH (LY; approx. 5 mg ml−1 in 0.1 m LiCl solution; 100-700 MΩ, mean ± s.d. = 360 ± 154 MΩ), ethidium bromide (EB; 6 mm in 1 m KCl; 60-509 MΩ, mean 127 ± 89 MΩ), or occasionally Cascade Blue (CB) at 30 mg ml−1 in 0.1 m LiCl solution (260-600 MΩ, mean 455 ± 120 MΩ). The microelectrode was advanced until a membrane potential was seen and an AP could be evoked by dorsal root stimulation with single rectangular pulses with durations of 0.03 ms (for A-fibre units) or 0.3 ms (for C-fibre units). Stimulus intensity was adjusted to twice threshold for A-fibre units and suprathreshold (usually 1-1.5 times threshold) for C-fibre units, and somatic APs were recorded on-line with a CED (Cambridge Electronics Design, Cambridge, UK) 1401 plus interface and the SIGAV program from CED and were subsequently analysed with a script running in the Spike II program (CED). The AP parameters measured were described previously (Djouhri et al. 1998) and include the AP duration at base (AP duration). The membrane potential was also recorded.
Conduction velocity (CV) was estimated from the latency to the rise of the dorsal root-evoked somatic AP, and the conduction distance (typically 4-7 mm) from the approximate (± 0.25 mm) location of the neurone in the DRG. The maximum measurement error of conduction distance was 10-15 % and utilisation time was not taken into account (for more details see Djouhri et al. 2001). CV ranges (C, Aδ and Aα/β) were based on dorsal root compound action potential waves (Djouhri et al. 1998), as follows: C < 1.1 m s−1, Aδ 1.1-4.2 m s−1 and Aα/β > 4.2 m s−1. Reasons for the relatively low CVs have been discussed previously (Lawson et al. 1997; Djouhri et al. 1998).
Sensory receptive properties
The sensory receptive fields of DRG neurones were on the ipsilateral hind limb and flank; their properties were examined and classified as previously described (Lawson et al. 1997; Djouhri et al. 1998). To summarise, units that responded to low intensity mechanical stimulation were categorised as low threshold mechanoreceptive (LTM) units. Most of these were cutaneous but Aα/β-fibre units with deeper receptive fields that responded to light pressure against muscle tissue, followed vibrations of 100 or 250 Hz, and often showed ongoing discharges, were classified as muscle spindle (MS) afferent units. This latter group included both Group I (Aα) and Group II (Aβ) categories and may have also included some Golgi tendon organ afferents. Low threshold C-fibre mechanoreceptive (C-LTM) units responded preferentially to slow gentle contact moving across the skin at < 1 mm s−1 and sometimes to cooling. Units that did not respond to low intensity stimuli but did respond to strong mechanical stimuli and lacked prompt responses to noxious heat were classified as high threshold mechanoreceptive (HTM) units. Some nociceptive units responded both to high intensity mechanical stimuli and also promptly to a single application of hot water at > 50 °C (noxious heat); these included A-mechano-heat (A-MH) units; C-polymodal (C-PM) units with superficial (probably epidermal or superficial dermal) receptive fields and C-mechanoheat (C-MH) units with receptive fields that were not superficial (presumed to be in dermis). The term ‘nociceptive units’ in this paper includes HTM as well as MH or C-PM units. Cooling units were those that responded to a brief spray of ethyl chloride, a cooled metal rod or ice and that often showed ongoing activity at room temperature, which ceased with slight warming.
Unresponsive units with A- or C-fibres were those for which no receptive field was found despite an extensive search over the whole hind limb with the usual non-noxious and noxious, mechanical and thermal stimuli.
Neuronal labelling
Once each unit was characterised, dye was ejected electrophoretically into the soma from the electrode by rectangular current pulses (1 nA for 500 ms at 1 Hz) for periods of up to 5 min. These currents were negative for LY and CB (0.6-1.0 nA min) and positive for EB (0.3-1 nA min). Usually two neurones at opposite ends of each DRG were injected with LY or CB, and one neurone in the mid-region was marked with EB. In this study, 44 cells were labelled with LY, 29 with EB and 5 with CB.
At the end of the experiment, the animals were killed under deep anaesthesia by perfusion through the heart with 0.9 % saline followed by Zamboni's fixative (Stefanini et al. 1967). Experimental DRGs were removed, post-fixed in Zamboni's, and soaked in 30 % sucrose buffer at 4 °C overnight. The next day, serial 7 μm cryostat sections of the DRGs were cut. Sections were mounted on 20 slides so that each slide had a series made up of every 20th section. Each section was examined under fluorescence microscopy; digital images of profiles of dye-labelled neurones were captured and camera lucida drawings made to ensure relocation of each profile after immunocytochemistry. The detailed position of every dye-filled cell recovered was compared to cell locations recorded during the experiment. If the expected and actual locations did not match, or if too many or too few cells were labelled, units were rejected. Tissue sections were stored at −20 °C until immunocytochemistry was carried out. Small ‘edge’ sections through a dye-labelled cell, that might not include the cell throughout the section thickness, were not used for immunocytochemistry.
Rat tissue
Cryostat sections (7 μm) of DRGs from rats that had been perfuse-fixed with Zamboni's fixative or 4 % paraformaldehyde under deep pentobarbitone anaesthesia were used for immunocytochemistry on rat tissue.
Immunocytochemistry
Endogenous biotin-like activity was blocked using the Vector SP-2001 blocking kit, prior to immunocytochemistry. Avidin-biotin immunocytochemistry (Vectastain Elite ABC kit: rabbit IgG, Vector Laboratories Ltd, UK) was performed, adding 0.3 % Triton X-100 to the washes, antibody and ABC steps. Sections were exposed to primary anti-Nav1.7 antibody at a concentration of 3 μg ml−1 in Tris-buffered saline with 0.3 % Triton X-100, for approximately 48 h at 4 °C. Any sections with generally poor immunocytochemistry were not used for further analysis.
Characterisation of the anti-Nav1.7 antibody on guinea-pig tissue
The primary polyclonal anti-Nav1.7 antibody had been prepared as previously reported (Toledo-Aral et al. 1997) by immunising rabbits with a synthetic peptide coupled to keyhole limpet haemocyanin, to residues 446-460 in rat Nav1.7, a sequence not present in other Na+ channel subunits. Because the antibody was raised against an epitope on rat Nav1.7, and the original antibody characterisations were done on rat tissue, we examined whether the staining in the guinea-pig was similar and therefore likely also to be specific for Nav1.7. In addition, the specificity of the antibody for Nav1.7 in guinea-pig DRG tissue was examined with preabsorption tests, using the peptide against which the antibody was raised, as follows. Sections from the same guinea-pig DRG were treated identically except for the primary antibody incubation, which was with one of the following: (a) antibody (3 μg ml−1) that had not been preabsorbed was applied to the section overnight at 4 °C (Fig. 1C); (b) Nav1.7 antibody (3 μg ml−1) preabsorbed by incubating overnight with peptide at a concentration of 5 × 10−5m, representing a 100 times molar excess of peptide over antibody, and then applied to the section overnight at 4 °C (Fig. 1D) or (c) no primary antibody (Fig. 1E). In addition, Western blots were carried out on both guinea-pig and rat DRG tissue with this antibody. Further confirmation of the specificity of this antibody was achieved by comparing staining patterns with a separate polyclonal antibody raised to a different epitope on Nav1.7.
Figure 1. Immunostaining of neurones to show Nav1.7 in rat and guinea-pig DRGs.
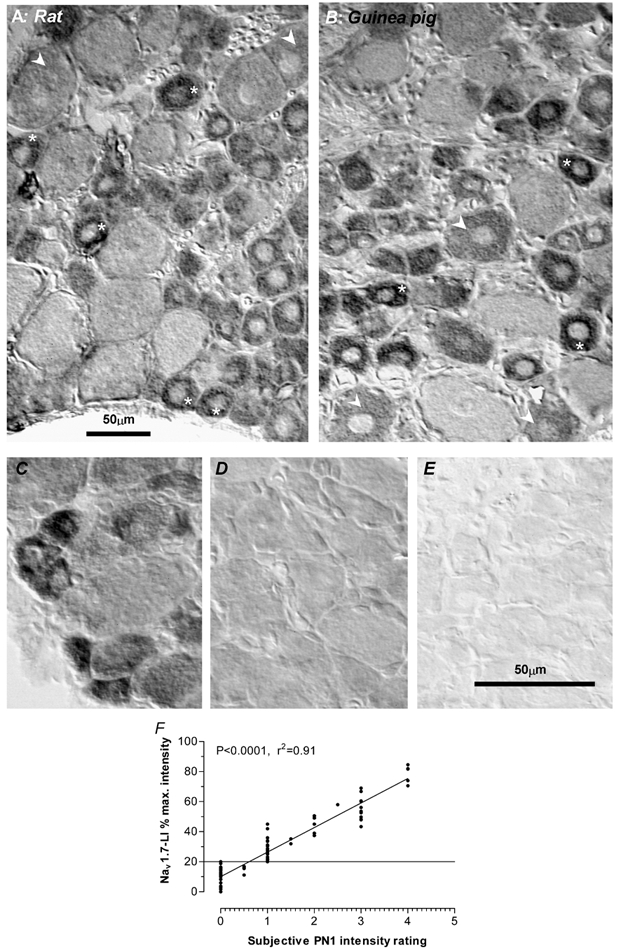
A and B compare the staining with anti-Nav1.7 antibody of rat (A) and guinea-pig (B) DRG sections on the same slide. The small cells were the most intensely stained (asterisks), medium sized cells often had an intermediate staining intensity (arrowheads) and the palest staining profiles were usually of large neurones. These images were captured under bright field optics with interference contrast to aid visualisation of the paler staining neurons, although image analysis was always carried out on bright field images. C, D and E, sections of the same guinea-pig L6 DRG on which immunocytochemistry was carried out simultaneously with the following primary antibody: C, normal anti-Nav1.7 antibody; D, anti-Nav1.7 antibody was pre-absorbed for 3 h prior to use with 5 × 10−5m of the peptide against which the antibody had been generated; E, no primary antibody. F, the relationship between subjectively judged relative intensity (abscissa) and the calculated relative intensity (ordinate) (see Methods); 20 % relative intensity or above was consistently judged to be clearly positive.
Analysis of Nav1.7-LI relative intensity
Dye-injected neurones stained to show Nav1.7-LI were each scored in two ways. The first scoring method was subjective by agreement between two observers as follows: 0 means negative, and 1-5 means clearly positive; 1 means weakly positive and 5 is equal to the most intensely stained neurone seen in the section. Secondly, a more objective semi-quantitative method of assessing relative intensity using image analysis was used. Images of cells were captured under standardised brightfield conditions using Openlab software (Improvision, Coventry, UK) and a cooled integrating CCD camera (Photonic Science, Robertsbridge, UK), and analysed with a program (macro) to run in NIH image. For each cell cytoplasm (excluding the nucleus), the mean value of the darkest 10 % of the pixels (from a frequency distribution histogram of pixel density) was taken as a measure of absorbance of light by the Nav1.7-LI reaction product. This method enables labelling that is punctate or globular to be clearly distinguished above background levels. Using these measures, the relative intensity (absorbance level) for each dye-injected cell was calculated as follows. To provide comparisons with the dye-labelled cell, the 0 % intensity level (a) was taken as the mean intensity of three clearly negative neuronal profiles near the labelled cell. The 100 % intensity level (b) was the mean intensity of the three most intensely stained profiles in the section. The intensity of the dye-labelled cell provided the value ‘c‘. The relative Nav1.7-LI intensity of the cell was calculated as (c – a)/(b – a) and expressed as a percentage. The calculated relative intensities and subjective relative intensity 0-5 ratings were highly correlated with an r2 value of 0.91, n = 78 (Fig. 1F). Because neurones with relative intensity > 20 % were consistently judged subjectively as being clearly positive by all viewers (see Fig. 1F), the term ‘positive’ refers to neurones with relative intensities > 20 %, and ‘negative’ refers to units with values < 20 %. We cannot exclude the possibility that cells with relative intensities < 20 % and classified as ‘negative’ may have had low levels of Nav1.7 protein. This method compensates for variability in immunocytochemical staining between sections and/or different immunocytochemical runs.
Cell size measurement
For each dye-labelled neurone, the cross sectional area of all the sections was measured and the largest of these sections (usually containing a clear nuclear profile) was used as the measure of cell size. Although this would be a perfect indicator of size for completely spherical neurones, not all neurones are spherical.
Sampling bias
The ease of making recordings from these neurones is greater for faster conducting neurones. This was offset to some extent by a conscious bias towards selecting neurones with slower CVs and/or nocicptive receptive fields. Nonetheless, the proportion of C-fibre neurones is considerably under-represented in this study.
Criteria for acceptance of data
Samples of each (sensory) type of DRG neurone were chosen for immunocytochemistry. This and the sampling bias mean that the proportions of cells in each group do not therefore provide an accurate indication of the proportions of different types of unit in the DRG. For the 78 units with successful immunocytochemistry, the mean animal weight was 220 ± 3.6 g (s.e.m.), range 167-300 g and the mean temperature at the DRG was 30 ± 0.12 °C (s.e.m.).
For the analysis of AP characteristics, cells were included only if (a) they had membrane potentials of −40 mV or more negative; (b) the temperature at the DRG was 28-31.5 °C; and (c) the somatic AP had an amplitude > 35 mV. The number of cells included in this analysis was 68.
Statistics
2 × 2 contingency tables and χ2 tests compared proportions of neurones (Fig. 3). The median relative intensities of groups shown in Fig. 4, except for the single C-LTM unit, were compared with the Kruskall-Wallis test (the non-parametric equivalent of one way ANOVA) followed by Dunn's post hoc test comparing all pairs of groups (Prism version 3, GraphPad Software Inc., USA). The median relative intensities of nociceptive and LTM units (Fig. 4) were compared with the Mann-Whitney test using Prism Software; linear regression analysis carried out on the data displayed in Fig. 5 is shown in Table 1. Where significant correlations existed on the same graph e.g. for nociceptors and LTM units, the regression lines were compared (Table 1) for slope, intercepts and elevation (Prism 3 software, also see Zar, 1984).
Figure 3. Comparison of the percentages of neurones with different CVs and sensory properties that show Nav1.7-LI.
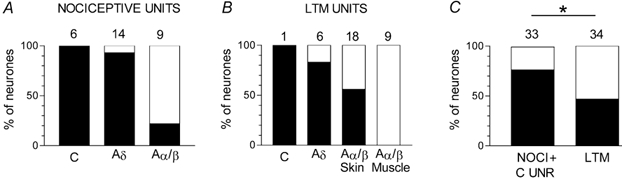
Graphs show the percentages of nociceptive (A) and LTM (B) units in the different CV ranges, and in nociceptive and LTM (low threshold mechanoreceptive) units in all CV groups together (C) that showed clear Nav1.7-LI (≥ 20 % relative intensity). It can be seen in A and B that these percentages were lower in groups with higher mean CVs. C, the percentage of nociceptive units that was positive was significantly greater than the percentage of LTM units that was positive (χ2 test, 2 × 2 contingency table). The number of units is shown above each column. NOCI, nociceptive, all conduction velocities; C UNR, C-fibre unresponsive units.
Figure 4. Relative intensities of Nav1.7-LI in neurones defined by sensory properties and CV.
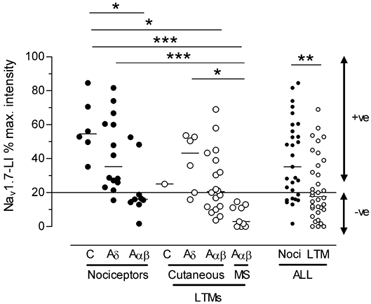
A scattergraph showing the distribution of relative intensities of Nav1.7-LI in the different groups of nociceptive and LTM (low threshold mechanoreceptive) neurones, and, in the last two columns, all nociceptive and all LTM neurones grouped together. •, nociceptors; ○, LTMs. Cutaneous LTMs includes Aδ (D hair) units and Aα/β (G hair, field, slowly adapting and rapidly adapting) units; MS, mainly muscle spindle, proprioceptive, afferent units. Medians are shown. The Kruskall-Wallis test with Dunn's post hoc test was used to compare all subgroups except C LTMs (only one cell). The Mann-Whitney test was used to compare medians of all nociceptive and all LTM units. Medians that were significantly different are indicated with a line above the two groups and asterisks show the level of significance *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5. Graphs of Nav1.7-LI intensity against neuronal properties.
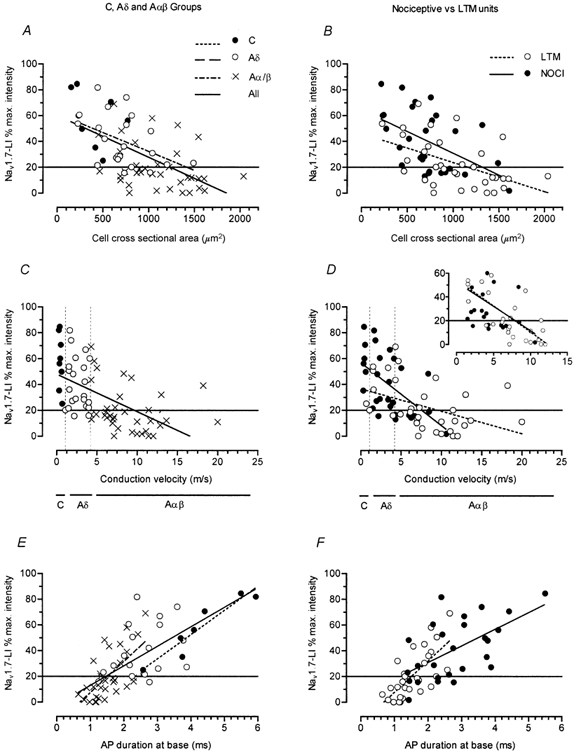
Neuronal properties plotted against Nav1.7-LI intensity are cell size (the cross-sectional area of the largest section through the neurone; A and B); dorsal root CV (C and D); and AP duration (the duration at base of somatic APs evoked by electrical stimulation of the dorsal root; E and F). The horizontal line at 20% indicates the relative intensity above which cells are considered positive for Nav1.7. The inset in D shows superimposition of the lines for nociceptive (NOCI) and low threshold mechanoreceptive (LTM) units when only units with CVs of 1.1-12 m s−1 were included (see text). On the left, units are subdivided into those with C- (•), Aδ- (○) or Aα/β-fibres (×); in these groups nociceptive and non-nociceptive units, and cooling-sensitive units were included. Unresponsive units were excluded. Linear regression analysis was carried out on each of these CV groups separately and on all units together, and regression lines are included only where there was a significant correlation. On the right, only nociceptive (•) and LTM units (○) are included; thus fewer units are plotted. Linear regression analysis was carried out on nociceptive and LTM units separately. The keys to symbols and lines may be found in A (for A, C and E) and B (for B, D and F). For significant linear correlations (P < 0.05), regression lines are shown here and the P and r2 values are shown in Table 1.
Table 1.
Correlations between Nav 1.7 relative intensity, cell size, CV and AP duration at base
| All | C | Aδ | Kδ/β | Noci | LTM | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | ± | r2 | n | P | ± | r2 | n | P | ± | r2 | n | P | ± | r2 | n | P | ± | r2 | n | P | ± | r2 | n | |
| Intensity vs. size | **** | — | 0.35 | 66 | n.s. | 9 | * | — | 0.2 | 22 | n.s. | 36 | ** | — | 0.25 | 29 | ** | — | 0.25 | 34 | ||||
| Intensity vs. CV | **** | — | 0.31 | 66 | n.s. | 9 | n.s. | 22 | n.s. | 36 | ** | — | 0.2 | 29 | ** | — | 0.19 | 34 | ||||||
| Intensity vs. APdB | **** | + | 0.53 | 57 | ** | + | 0.89 | 7 | n.s. | 19 | *** | + | 0.38 | 31 | ** | + | 0.35 | 26 | **** | + | 0.50 | 29 | ||
| APdB vs. CV | **** | — | 0.36 | 57 | n.s. | 7 | n.s. | 19 | n.s. | 31 | *** | — | 0.48 | 26 | ** | — | 0.22 | 29 | ||||||
| APdB vs. size | *** | — | 0.34 | 57 | n.s. | 7 | n.s. | 19 | n.s. | 31 | * | — | 0.23 | 26 | * | — | 0.20 | 29 | ||||||
For linear correlations shown in Fig. 5 (upper section of table), and for correlations between CV, cell size and AP duration (lower section of table), the following are shown: P values, whether correlations are positive (+) or negative (—), r2 values and the number of units, n. In the first column, “intensity” means relative intensity of Nav1.7-LI, “size” means cell cross-sectional area through the largest section in that cell and “APdB” means AP duration at base. Units were included for CV and cell size regardless of membrane potential or AP height. Selection criteria for cells for including their AP durations are given in the text. The same data were subdivided according to CV (columns 3–5) and then according to sensory properties (see legend to Fig. 5). The regression lines for nociceptive and LTM units were compared (GraphPad Prism software). In no case was there a significant difference in the slopes or elevations of these lines.
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
n.s., not significant.
Possible sources of error
The problems with locating dye-injected cells have been reported in detail (Lawson et al. 1997), and all precautions necessary to avoid the problem of cells being inadvertently labelled by dye leakage from the electrode that were outlined in that paper were followed in the present study. The weight range of the animals and the temperature at the DRG were controlled within narrow limits. Minor sources of error in CV are described above.
Results
The staining pattern in the rat (Fig. 1A) was indistinguishable from that in the guinea-pig (Fig. 1B), with smaller DRG neurones being more intensely stained than large neurones, and with some large neurones weakly stained or unstained. Preabsorption of the anti-Nav1.7 antibody led to virtually complete loss of staining, leaving a level of background staining that was somewhat higher than when no primary antibody was included, both in guinea-pig (Fig. 1C, D and E) and rat DRG sections (not shown). This Nav1.7 antibody specifically detected a protein of an appropriate size following Western blot analysis of rat DRG membrane extract (Toledo-Aral et al. 1997). A similar result was obtained here with Western blots (not shown) of both guinea-pig and rat DRG protein preparations. In addition, another Nav1.7 antibody raised against a different epitope showed similar staining to the present antibody in rat DRGs, confirming the preferential small cell labelling. There is therefore good evidence that the antibody we used labels Nav1.7 protein, preferentially in smaller neurones, in both rat and guinea-pig DRGs.
Nav1.7-LI was examined in 78 DRG neuronal somata with identified sensory properties. These included 12 C-fibre units (6 nociceptive, 1 C-LTM, 4 unresponsive and 1 C-cooling units), 27 Aδ-fibre units (14 nociceptive, 6 D hair LTM, 1 cooling unit (see below), 1 moderate pressure that also fired to cooling and 5 unresponsive units), and 39 Aα/β-fibre units (9 nociceptive, 27 LTM units including 9 muscle spindle and 18 cutaneous LTMs, and 3 unresponsive units). The Aδ moderate pressure unit was not included in nociceptive or LTM groups as it had sensory receptive and electrophysiological properties intermediate between the two. The cooling Aδ unit fired spontaneously at room temperature, and responded to a cooled metal spatula applied to hairy skin but not to purely mechanical stimuli. The C-fibre unresponsive units had very long AP and AHP durations, indicating (see Djouhri & Lawson, 1998) that they were ‘nociceptive-type’ neurones, likely to be either very high threshold nociceptive neurones (the so-called ‘silent nociceptors’) or nociceptive units with inaccessible receptive fields. However, many of the A-fibre unresponsive neurones had AP and AHP durations consistent with some of them being LTM units with inaccessible receptive fields. These A-fibre unresponsive units were therefore not included in later analyses, as their identity was unclear. Examples of Nav1.7-LI in identified units in Fig. 2 include a C-PM unit with intense staining, two Aδ units both with moderate staining intensities (a D hair LTM and an Aδ-nociceptive unit) and a muscle spindle afferent unit which is negative (< 20 % relative absorbance).
Figure 2. Photomicrographs of dye-injected, physiologically identified neurones.
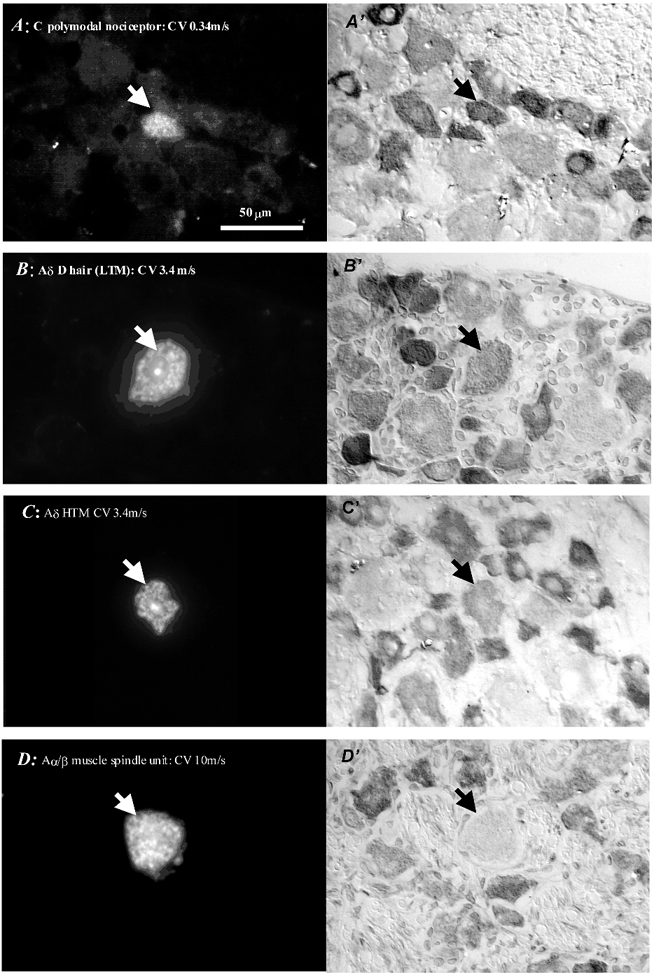
On the left are the fluorescence photomicrographs of sections through neurones indicated by the arrow in each image injected with Lucifer yellow (A) or ethidium bromide (B, C and D). On the right (A‘-D‘) are the same neurones after immunocytochemistry to show Nav1.7-LI; these images were captured under interference contrast optics to increase clarity, but note that all image analysis was performed on bright field images. Sections of the dye-injected neurone in A, B and C, but not D, were through the nucleus. The C polymodal nociceptor in A shows intense labelling; both Aδ-fibre units (a D hair LTM unit in B and a nociceptive unit in C) show intermediate labelling. The muscle spindle afferent in D shows weak or absent labelling and, having no nucleus, does not indicate the full size of the cell. LTM, low threshold mechanoreceptor; HTM, high threshold mechanoreceptor.
Of the 68 units that fulfilled the criteria for the AP analyses, 10 were C-fibre units (5 nociceptive, 1 C-LTM, 1 C cooling and 3 unresponsive), 24 were Aδ-units (14 nociceptive, 4 D hair LTM, the moderate pressure unit that fired to cooling and 5 unresponsive units), and 34 were Aα/β units (7 nociceptive, 24 LTM units and 3 unresponsive units).
Comparisons of the proportions of units that were clearly positive (relative intensity ≥ 20 %) for Nav1.7 are shown in Fig. 3. All C-, 93 % Aδ- and 22 % Aα/β-fibre nociceptive units were Nav1.7-LI-positive. However, LTM units were also positive including one weakly positive C-LTM unit, most (83 %) D hair units, 56 % of the cutaneous Aα/β LTM units but none of the muscle spindle LTM units. Thus positive units included both nociceptive and LTM units. Overall, however, the proportion of all nociceptive units, including C-unresponsive (i.e. nociceptive-type) units, that showed Nav1.7-LI (72.4 %) was significantly greater than the proportion of LTM units that was positive (47 %), Fig. 3C.
There is a wide range of relative intensities of Nav1.7-LI in the different groups of DRG neurones (Fig. 4). Despite this range, statistical differences (Kruskall-Wallis test) existed between the medians of some groups, which were generally higher in the groups with slower CVs. The median for all nociceptive units was significantly greater than that for all LTM units (Mann-Whitney test). There was no clear difference in intensity between C-HTMs and C-PMs (not shown). Of the 15 units not included in Fig. 4, a C-cooling unit (CV 0.27 m s−1) was intensely labelled (82 % relative intensity), four unresponsive C-fibre units were clearly positive (31-58 %), the Aδ-cooling unit and the moderate pressure unit were weakly positive (31 and 22 % respectively), five Aδ unresponsive units were all positive (20-51 %) but three unresponsive Aα/β-fibre units were negative. Thus in the unresponsive units, as well as the nociceptive and LTM units, those with the more slowly conducting fibres showed the highest relative intensities. These patterns, including the lack of staining in the muscle spindle afferents which tend to have CVs in the high Aα/β range, appeared to indicate that staining intensity may be greater in units with low CVs.
The relative intensity of Nav1.7-LI was correlated negatively with cell size for all cells, Aδ neurones (weakly) and for both nociceptive and LTM neurones (Fig. 5A and B, Table 1). The greater association of Nav1.7 intensity with nociceptive units may therefore in part be secondary to its relationship with cell size, due to the smaller mean size of nociceptive units (see Fig. 5B).
The Nav1.7 intensity was also negatively correlated with dorsal root CV for all units together (Fig. 5C), and for nociceptive and LTM neurones separately (Fig. 5D). The apparent difference in slopes of the nociceptive and LTM regression lines was not significant, and appears to be due to the greater preponderance of C-fibre units with nociceptive properties and fast conducting Aα/β-fibre units (> 12 m s−1) with LTM properties. This view is supported by the almost perfect superimposition of the regression lines for nociceptive and LTM units (see Fig. 5D insert) when these extreme ends of the range were excluded and only units with CVs of 1.1-12 m s−1 were compared (see inset). In this range there were significant correlations for both LTMs (P < 0.001, r2 = 0.48) and nociceptors (P < 0.05, r2 = 0.2). At least over this range, Nav1.7-LI relative intensity therefore appears to be more closely associated with CV than with sensory receptive properties, although with relatively low r2 values (Table 1). The greater association of Nav1.7 intensity with nociceptive units may therefore, in part, be secondary to its relationship with CV, due to the overall lower CV of nociceptive units.
Nav1.7-LI relative intensity showed significant positive correlations with AP duration at base for all neurones together, and for C and Aα/β units (Fig. 5E). The r2 value for C-fibre neurones with identified receptive fields was high (Table 1); units included five nociceptors, a C-LTM and a C-cooling unit. The r2 value was high even if only the nociceptive C-fibre units were included (P = 0.02, r2 = 0.86, n = 5). However, the two C-unresponsive neurones that had membrane potentials of at least −40 mV did not fall near this line, having longer APs (7.8 and 10.8 ms) than the nociceptive units but only intermediate staining (49 and 31 % respectively). Figure 5F shows that the regression line for LTM units extended more to the lower left, and the line for nociceptive neurones continued more to the upper right of the graph. The tendency of nociceptive neurones to have longer AP durations than LTM neurones within the same CV group has previously been demonstrated (Ritter & Mendell, 1992; Djouhri et al. 1998). The pattern seen here appears to indicate that there is a stronger relationship of Nav1.7-LI with AP duration than with sensory properties per se, and indeed that the more intense Nav1.7-LI in nociceptive units appears to be secondary to their longer AP duration.
Of the above correlations, that between Nav1.7-LI and AP duration at base was the strongest with the greatest r2 value, and indeed this was the only variable that showed a significant correlation with Nav1.7-LI within the C- and Aα/β-fibre groups. To examine the possibility that the correlations of Nav1.7-LI with cell size and CV could have been secondary to the correlation with AP duration at base, linear regression analysis between AP duration and both cell size and CV was carried out on the same cells, see Table 1. Significant correlations were found between both pairs of variables for all cells together, and for all nociceptive and for all LTM neurones separately. Indeed, the correlations between intensity and AP duration at base, and between AP duration at base and CV may be to some extent responsible for the weaker correlation between intensity and CV.
Discussion
Nav1.7-LI was more intense in smaller neurones, and in neurones with slower CVs. It was not limited to nociceptive neurones, being present also in LTM neurones, although overall a higher proportion of nociceptive than of LTM units were positive. Nav1.7-LI intensity was more strongly correlated with somatic AP duration than with cell size or CV.
Preferential labelling of small DRG neurones was seen in the rat and guinea-pig with this well characterised antibody. It was also seen in the rat with an antibody raised to a different epitope on Nav1.7, and with a further antibody that showed ‘labelling of all cell types but with an increased intensity in small diameter cells’ (Porreca et al. 1999).
Lack of membrane labelling
Na+ channel immunostaining has been observed using sensitive immunofluorescence and confocal microscopy methods in sites of very high Na+ channel density e.g. node of Ranvier or, with only weak confocal staining, in the postsynaptic membrane of skeletal muscle (Wood et al. 1998; Caldwell et al. 2000; Caldwell & Levinson, unpublished). However, in the DRG somata the channel density is order/s of magnitude lower than in these sites (see Cummins & Waxman 1997, Schild & Kunze, 1997) and was not previously seen in the DRG soma membrane even using the most sensitive immunofluorescence techniques (e.g. see Toledo-Aral et al. 1997; Gould et al. 2000). Indeed, other studies have shown (Brismar & Gilly, 1987; Thornhill & Levinson, 1992; Ukomadu et al. 1992) that the cytoplasmic pool of Na+ channel is usually much greater than that inserted into the soma membrane. It is therefore not surprising that it was not detectable in DRG cell membranes in this study, especially since a less sensitive non-fluorescence immunocytochemistry was employed. The pool of cytoplasmic protein in the DRG is presumably the source of the soma membrane channel protein, although much of it must be destined for the fibres whose surface areas are orders of magnitude greater than those of the soma. The correlations seen here between cytoplasmic Nav1.7-LI and soma membrane properties appear to indicate that the amount of functional protein in the soma membrane is related to the amount of cytoplasmic channel protein despite being probably only a small proportion of the total soma channel protein.
Nav1.7 protein versus mRNA
In adult rat DRGs, Nav1.7 protein (Porreca et al. 1999; Gould et al. 2000) is more highly expressed in small than large neurones despite the presence of mRNA in neurones of all sizes (Black et al. 1996). In large neurones, the low protein levels may reflect (a) poor translational efficiency; (b) post-translational modification and/or (c) rapid transport from the somata. The lack of Nav1.7-LI in large myelinated peripheral fibres (S.R. Levinson, unpublished observations) makes (c) unlikely. However, Nav1.7-LI at nerve terminals in cultured E15 DRG neurones (Toledo-Aral et al. 1997) and clear Nav1.7-LI in most unmyelinated (C-) fibres (S. R. Levinson, unpublished observations) does suggest transport of protein to certain fibres and terminals. Present findings of somata with C-fibres having the highest Nav1.7-LI support the view that staining intensity of the soma is directly related to that of the fibre.
Sensory receptive properties
In contrast to the pattern of expression of the TTX-R Na+ channel α subunit Nav1.9 which is expressed exclusively in nociceptive-type DRG neurones (Fang et al. 2002), Nav1.7 is expressed in both nociceptive and LTM neurones. Thus a marker associated mainly with small neurones may (e.g. Nav1.9) or may not (e.g. Nav1.7) be exclusively associated with nociceptive neurones. Nonetheless, even Nav1.7 was in a higher proportion of nociceptive than LTM units, with higher median staining intensity in nociceptive units. Indeed, we would expect the proportion of nociceptive units that are positive would have been even greater if small neurones with slow CVs had not, as usual in this type of study, been under-represented.
Action potential duration
The clear relationship between Nav1.7 and AP duration especially in C-fibre neurones indicates a relationship between cytoplasmic Nav1.7 protein levels and levels of functional channel protein in the soma membrane. There is a similarity in the pattern of both Nav1.7-LI intensity and AP duration at base in relation to sensory properties and CV, which is also consistent with a functional link between Nav1.7-LI and AP duration. Both are graded with CV, being most extreme (i.e. most intense Nav1.7-LI and longest AP durations) in nociceptive units with slow CVs, and less extreme but with a similar graded pattern with CV in LTM neurones. This pattern of nociceptive properties was classed as pattern A (Lawson, 2002).
Possible explanations for slower AP kinetics being linked with more Nav1.7 protein include (a) the possibility of slower activation/inactivation kinetics for Nav1.7 than for the other TTXS subunits that dominate the Na+ channel complement in larger DRG neurones (Cummins et al. 1998); (b) co-expression with other subunits that results in slow AP kinetics (e.g. Nav1.8); (c) influence of Nav1.7 on the kinetics of other Na+ channel subunits. The probable complexities of interaction between channel subtypes make it impossible to speculate further, although the data do support a role for Nav1.7 in generation of the AP inward current.
Conduction velocity
The stronger correlation of somatic Nav1.7-LI intensity with somatic AP duration than with fibre CV, taken with the correlation between soma AP duration and fibre CV, may indicate that the relationship between Nav1.7-LI and CV is secondary to that with soma AP duration. This would make sense if functional channel subtypes in soma and fibre membranes are similar, since slower AP rise times in the fibre would result in slower conduction along the fibre.
Clinical relevance
Nav1.7 may not, at first sight, appear a good choice as a target for a Na+ channel-directed novel analgesic drug due to its expression in LTM neurones and in sympathetic neurones which may lead to unwanted side effects. Nonetheless, the present data may indicate that targeting this subunit could influence nociceptive more than non-nociceptive DRG neurones. More information is needed about its role especially in models of chronic pain before it can usefully be assessed as a possible therapeutic target.
In summary, although Nav1.7 is not limited to nociceptive neurones, it is higher in DRG neurones with broader somatic APs that have slower CVs and smaller somata. These properties are associated with nociceptive neurones, which may account for the overall greater expression of Nav1.7 in nociceptive than LTM neurones. Targeted manipulation of Nav1.7 expression followed by examination of membrane function in identified neurones would help to determine the contribution of Nav1.7 to DRG membrane properties.
Acknowledgments
This work was supported by a Wellcome Trust grant to S.N.L. and NIH grant NS34375 to S.R.L. We are grateful to Ken Salisbury of Improvision for the program to run in NIH Image.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Zerillo CA, Levinson R, Ritchie JM, Howe JR. Cloning of a sodium channel alpha subunit from rabbit Schwann cells. Proc Natl Acad Sci U S A. 1995;92:11034–11038. doi: 10.1073/pnas.92.24.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurones express multiple sodium channel alpha-subunit mRNAs. Brain Res Mol Brain Res. 1996;43:117–131. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Brismar T, Gilly WF. Synthesis of sodium channels in the cell bodies of squid giant axons. Proc Natl Acad Sci U S A. 1987;84:1459–1463. doi: 10.1073/pnas.84.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1. 6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Howe JR, Waxman SG. Slow closed-state inactivation: a novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J Neurosci. 1998;18:9607–9619. doi: 10.1523/JNEUROSCI.18-23-09607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurones after nerve injury. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci U S A. 1998;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurons. J Physiol. 1998;513:857–872. doi: 10.1111/j.1469-7793.1998.857ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Increased conduction velocity of nociceptive primary afferent neurones during unilateral hindlimb inflammation in the anaesthetised guinea-pig. Neurosci. 2001;102:669–679. doi: 10.1016/s0306-4522(00)00503-0. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Levinson SR, Lawson SN. Sensory receptive properties and conduction velocities of dorsal root ganglion neurones that express the PN1 Na+ channel subunit. Soc Neurosci Abst. 2000;26:352. [Google Scholar]
- Fang X, Djouhri L, Black JA, Dib-Hajj S, Waxman SG, Lawson SN. The presence and role of the TTX resistant sodium channel Nav1. 9 (NaN) in nociceptive primary afferent neurons. J Neurosci. 2002;22:7427–7433. doi: 10.1523/JNEUROSCI.22-17-07425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, III, Gould TN, England JD, Paul D, Liu ZP, Levinson SR. A possible role for nerve growth factor in the augmentation of sodium channels in models of chronic pain. Brain Res. 2000;854:19–29. doi: 10.1016/s0006-8993(99)02216-7. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Mense S, Scherotzke R. Calcitonin gene-related peptide immunoreactivity in functionally identified primary afferent neurones in the rat. Anat Embryol. 1994;189:41–49. doi: 10.1007/BF00193128. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Flockerzi V, Hofmann F. Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J. 1995;14:1084–1090. doi: 10.1002/j.1460-2075.1995.tb07091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN. Phenotype & function of somatic primary afferent nociceptive neurones with C-, Aδ or Aα/β-fibres. J Exp Physiol. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER. Relationship of Substance P to afferent characteristics of dorsal root ganglion neurones in guinea pig. J Physiol. 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Lai J, Bian D, Wegert S, Ossipov MH, Eglen RM, Kassotakis L, Novakovic S, Rabert DK, Sangameswaran L, Hunter JC. A comparison of the potential role of the tetrodotoxin-insensitive sodium channels, PN3/SNS and NaN/SNS2, in rat models of chronic pain. Proc Natl Acad Sci U S A. 1999;96:7640–7644. doi: 10.1073/pnas.96.14.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: Effects of nerve growth factor. J Neurophys. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Sangameswaran L, Fish LM, Koch BD, Rabert DK, Delgado SG, Ilnicka M, Jakeman LB, Novakovic S, Wong K, Sze P, Tzoumaka E, Stewart GR, Herman RC, Chan H, Eglen RM, Hunter JC. A novel tetrodotoxin-sensitive, voltage-gated sodium channel expressed in rat and human dorsal root ganglia. J Biol Chem. 1997;272:14805–14809. doi: 10.1074/jbc.272.23.14805. [DOI] [PubMed] [Google Scholar]
- Schild JH, Kunze DL. Experimental and modeling study of Na+ current heterogeneity in rat nodose neurons and its impact on neuronal discharge. J Neurophysiol. 1997;78:3198–3209. doi: 10.1152/jn.1997.78.6.3198. [DOI] [PubMed] [Google Scholar]
- Stefanini M, De Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967;216:173–175. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Tate S, Benn S, Hick C, Trezise D, John V, Mannion RJ, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf CJ. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- Thornhill WB, Levinson SR. Biosynthesis of ion channels in cell-free and metabolically labeled cell systems. Methods Enzymol. 1992;207:659–670. doi: 10.1016/0076-6879(92)07047-r. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Moss BL, He ZJ, Koszowski AG, Whisenand T, Levinson SR, Wolf JJ, Silos-Santiago I, Halegoua S, Mandel G. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci U S A. 1997;94:1527–1532. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzoumaka E, Novakovic SD, Haraguchi M, Sangameswaran L, Wong K, Gogas KR, Hunter JC. PN3 sodium channel distribution in the dorsal root ganglia of normal and neuropathic rats. Proc West Pharmacol Soc. 1997;40:69–72. [PubMed] [Google Scholar]
- Ukomadu C, Zhou J, Sigworth FJ, Agnew WS. mul Na+ channels expressed transiently in human embryonic kidney cells: biochemical and biophysical properties. Neuron. 1992;8:663–676. doi: 10.1016/0896-6273(92)90088-u. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Shewry K, Young C, Slater CR. An early stage in sodium channel clustering at developing rat neuromuscular junctions. Neuroreport. 1998;9:1991–1995. doi: 10.1097/00001756-199806220-00014. [DOI] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. 2. Englewood Cliffs, NJ, USA: Prentice Hall; 1984. [Google Scholar]


