Abstract
The cytokine interleukin (IL)-6 has recently been linked with type 2 diabetes mellitus and has been suggested to affect glucose metabolism. To determine whether acute IL-6 administration affects whole-body glucose kinetics or muscle glucose uptake, 18 healthy young men were assigned to one of three groups receiving a high dose of recombinant human IL-6 (HiIL-6; n = 6), a low dose of IL-6 (LoIL-6; n = 6) or saline (Con; n = 6) infused into one femoral artery for 3 h. The stable isotope [6,6-2H2] glucose was infused into a forearm vein throughout the 3 h infusion period and for a further 3 h after the cessation of infusion (recovery) to determine endogenous glucose production and whole-body glucose disposal. Infusion with HiIL-6 and LoIL-6 resulted in a marked (P < 0.05) increase in systemic IL-6 concentration throughout the 3 h of infusion (mean arterial plasma [IL-6]s of 319 and 143 pg ml−1 for HiIL-6 and LoIL-6, respectively), followed by a rapid decline (P < 0.05) during the recovery period. Subjects experienced clinical symptoms such as shivering and discomfort during HiIL-6 administration, but were asymptomatic during LoIL-6 administration. In addition, only HiIL-6 elevated (P < 0.05) plasma adrenaline (epinephrine). IL-6 infusion, irrespective of dose, did not result in any changes to endogenous glucose production, whole-body glucose disposal or leg- glucose uptake. These data demonstrate that acute IL-6 administration does not impair whole-body glucose disposal, net leg-glucose uptake, or increase endogenous glucose production at rest in healthy young humans.
Elevated plasma levels of the cytokines tumour necrosis factor-α and interleukin (IL)-6 have been consistently observed in patients with type 2 diabetes mellitus, and as a consequence both have been related to insulin resistance and/or impaired glucose disposal (for review see Hotamisligil, 1999; Febbraio & Pedersen, 2002). Research from our laboratory has focussed on the effect of muscle contraction on the IL-6 response (for review see Pedersen et al. 2001). We have demonstrated that contracting muscle releases IL-6, and that this release can account for much of the systemic increase in [IL-6] found in response to exercise (Steensberg et al. 2000), even though the brain (Nybo et al. 2002) and the peritendon (Langberg et al. 2002) can release small amounts of IL-6 during exercise. In addition, IL-6 mRNA is upregulated in contracting muscle (Keller et al. 2001; Starkie et al. 2001; Steensberg et al. 2001). Of note, IL-6 mRNA expression, the nuclear transcriptional rate of IL-6, and release of this protein from human skeletal muscle are enhanced when exercising with low intramuscular glycogen stores (Keller et al. 2001; Steensberg et al. 2001). In addition, Helge et al. (2002) recently demonstrated that IL-6 release was positively correlated with leg-glucose uptake during graded exercise. Thus, IL-6 appears to be upregulated in the muscle when the requirement for glucose uptake is augmented. Hence, rather than indicate that IL-6 impairs glucose uptake, our recent data suggest that IL-6 levels are augmented during periods of enhanced glucose uptake, although it must be noted that IL-6 release occurs after 60 min of exercise, whereas glucose uptake is increased at the onset of muscle contraction (Steensberg et al. 2001).
Recently, Wallenius et al. (2002) demonstrated that IL-6-deficient mice developed markedly impaired glucose disposal, compared with littermate control mice during an intravenous glucose tolerance test. In addition, Stouthard et al. (1996) demonstrated that IL-6 enhanced both basal and insulin-stimulated glucose uptake in cultured 3T3-L1 adipocytes, while Hardin et al. (2000) observed increased glucose transport in jejunal tissue incubated with IL-6 compared with controls. The effect of acute IL-6 administration on whole-body glucose metabolism is unclear. Tsigos et al. (1997) demonstrated that recombinant human IL-6 (rhIL-6) administration to healthy volunteers increased circulating plasma glucose in a dose-dependent manner. Although these authors could not determine whether rhIL-6 resulted in increased glucose production or decreased glucose disposal, they speculated that IL-6 might have produced peripheral resistance to insulin action. This hypothesis is plausible given the fact that acute rhIL-6 infusion increases circulating free fatty acids (FFA; Lyngsøet al. 2002), which according to the classic glucose-fatty acid cycle can lead to impaired glucose disposal (Randle et al. 1963). In contrast, Stouthard et al. (1995) studied patients with metastatic renal cell cancer receiving rhIL-6 infusion, and observed an increase in glucose appearance and whole-body glucose disposal using the isotopic tracer dilution method. Importantly, however, in this study (Stouthard et al. 1995) all subjects experienced clinical symptoms, such as fever, and consequently increased their whole-body oxygen consumption (V̇O2). In addition, circulating levels of hormones such as glucagon, adrenaline (epinephrine) and noradrenaline (norepinephrine) were elevated. Therefore, the authors could not determine whether the enhanced glucose disposal was a direct effect of IL-6, whether skeletal muscle was the site of the enhanced disposal, or whether the response was characteristic of healthy humans.
Hence, the aim of the present study was to investigate the role of acute IL-6 administration on whole-body glucose disposal and leg-glucose uptake in healthy humans by using the isotopic tracer dilution method and arterial- femoral venous (a-fv) differences across the legs. In order to determine whether any effect of IL-6 was direct, or secondary to changes in energy turnover and/or regulatory hormones, we chose to infuse both a low and a high dose of rhIL-6. The low dose elicited plasma concentrations of IL-6 comparable with the highest concentrations observed during prolonged strenuous exercise (Pedersen et al. 2001). We hypothesized that IL-6 infusion would increase glucose uptake by the muscle.
METHODS
Subjects
Eighteen healthy, active but not specifically trained males were recruited in the study. Each was assigned to one of three groups; control (Con: age 23 ± 1 years; body mass 78 ± 3 kg; height 183 ± 4 cm; body mass index (BMI) 23.4 ± 0.5 kg m−2), low rhIL-6 infusion (LoIL-6: age 24 ± 1 years; body mass 80 ± 2 kg; height 184 ± 4 cm; BMI 23.5 ± 0.8 kg m−2) and high IL-6 infusion (HiIL-6: age 26 ± 1 years; body mass 77 ± 2 kg; height 178 ± 2 cm; BMI 24.3 ± 0.7 kg m−2). The study was approved by the Ethical Committee of Copenhagen and Frederiksberg Communities, Denmark, and performed according to the Declaration of Helsinki. Subjects were informed about the possible risks and discomfort involved before giving their written consent to participate.
Protocol
Subjects reported to the laboratory at 07.00 h after an overnight fast. They voided, changed into appropriate hospital attire and remained supine during the entire experiment. They were only permitted to consume water during the experiment. After 10 min, the femoral artery and vein of both legs were cannulated as described previously (Steensberg et al. 2001). Thereafter, one catheter was placed in a forearm vein for infusion of the stable isotopes. One femoral arterial catheter was used for the infusion of IL-6 (or saline). Hence, one leg received the IL-6 infusion dose, and the other the systemic IL-6 concentration. The other arterial and two femoral venous lines were used for blood sampling. At each sampling time point the femoral arterial blood flow in both legs was measured with ultrasound Doppler flowmetry, as described previously (Rådegran, 1997). Multiple measures were made at each sampling point and the coefficient of variation for this technique has been determined to be ∼ 6 % (Rådegran, 1997). This allowed us to calculate any net changes across the legs by multiplying the a-fv differences with the blood flow using Fick's principle.
At ∼08.00 h the infusion of the stable isotopes commenced. Two hours later, when steady state was achieved, the IL-6 (or saline) infusion commenced. Muscle biopsy samples were obtained after application of a local anaesthetic (lidocaine (lignocaine) 20 mg ml−1) from the vastus lateralis of both limbs using the percutaneous needle biopsy technique with suction, 30 min before the commencement of the infusion, 30 and 90 min into the infusion, at the cessation of the infusion, and 180 min and 24 h following the infusion.
IL-6 infusates
The two different concentrations of rhIL-6 (Sandoz, Basle, Switzerland) were infused in doses lower than those reported to be safe in other studies (Stouthard et al. 1995). The IL-6 doses were chosen on the basis of pilot experiments. We aimed to reach plasma levels of IL-6 characteristic of intense prolonged exercise or low-grade inflammation during LoIL-6. In this trial, the rate of rhIL-6 infusion was 30 μg h−1, using saline as a vehicle. Due to the low bioavailability of IL-6 administered in saline, we could not use this method during HiIL-6. Hence, further pilot experiments were conducted using human albumin as a vehicle. These pilot experiments revealed that a dose of 15 μg h−1 was required during this trial. Saline was infused during control infusion (Con).
Blood analysis
Blood samples for IL-6 were measured by high-sensitivity enzyme-linked immunosorbent assay, as described previously (Steensberg et al. 2001). Plasma insulin (Insulin RIA 100, Amersham Pharmacia Biotech, Uppsala, Sweden), glucagon (Linco Research, St Charles, USA) and cortisol (Diagnostic Products Corporation, Los Angeles, USA) were analysed by radioimmunoassay, and plasma adrenaline and noradrenaline by high-performance liquid chromatography. These analyses are described in detail elsewhere (Blomstrand & Saltin, 1999; Steensberg et al. 2002). Plasma glucose was determined using an automatic analyser (Cobas Fara, Roche, France). Furthermore, an aliquot of blood was mixed with lithium heparin and spun in a centrifuge (2200 g for 15 min at 4 °C). The resultant plasma was stored for measurement of [6,6-2H2] glucose enrichment, as described previously (Steensberg et al. 2002).
Muscle analysis
Muscle biopsy samples were divided into two pieces upon sampling, and then frozen in liquid nitrogen for subsequent analysis. One portion was freeze-dried, dissected free from visible blood and connective tissue, extracted and analysed for glycogen. The second portion of the muscle biopsy sample was extracted for total RNA and analysed for GLUT 4 mRNA by Northern blot analyses. These procedures are described in detail elsewhere (Steensberg et al. 2002).
Physiological variables
Heart rate (HR) and blood pressure (BP) were measured every 60 min using electrocardiography and sphygmomanometry, respectively. Temperature (T) was also measured at this time point via a tympanic probe. Expired pulmonary gases were collected and analysed for V̇O2 on-line using a Medgraphics CPX/D metabolic cart (St Paul, MN, USA).
Statistics
All data are presented as means ±s.e.m. (n = 6). Log plasma [IL-6], GLUT 4 mRNA and glucagon were normally distributed; therefore the log-transformed values were used for the statistical analyses. To analyse changes over time and between groups, a two-way repeated-measures analysis of variance (RM-ANOVA) was used. If such analysis revealed significant differences, a Newman-Keuls post hoc test was used to locate the specific differences. The net leg-glucose uptake was analysed by a Friedman non-parametric test, because a normal distribution could not be obtained. P < 0.05 was accepted as significant.
RESULTS
There were no significant differences between the trials for HR, T or BP (data not shown). Due to technical difficulties we were unable to sample expired pulmonary gases during HiIL-6. There were no differences in V̇O2 when comparing Con with LoIL-6 (data not shown). The subjects did not report any discomfort during Con or LoIL-6. During HiIL-6 all subjects experienced approximately 30 min of shivering and discomfort 30–60 min after the IL-6 infusion was initiated. This effect was transient and none of the subjects reported any severe side effects.
The arterial plasma [IL-6] is shown in Fig. 1A. During LoIL-6 and HiIL-6, the mean arterial plasma [IL-6]s were 143 and 319 pg ml−1, respectively. Saline infusion did not increase the concentration of IL-6 in the venous effluent from the infusion leg (Fig. 1B). In contrast, IL-6 concen-tration in the venous blood from the infusion leg was elevated (P < 0.05) compared with pre-infusion concentrations in LoIL-6. This effect was markedly augmented during HiIL-6 (P < 0.05). There was a net disappearance of IL-6 from the plasma across the leg receiving the systemic IL-6 dose in both LoIL-6 and HiIL-6 (P < 0.05) (Fig. 1C).
Figure 1. Effect of infusion of high and low doses of recombinant human interleukin-6 (rhIL-6) on arterial and venous plasma [IL-6] and net differences in [IL-6].
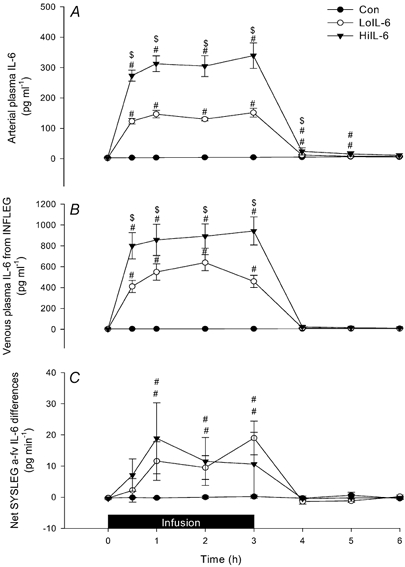
Arterial plasma [IL-6 ](A), venous plasma [IL-6] from the infusion leg (INFLEG) (B) and net [IL-6] differences in the systemic leg (SYSLEG) (C) during and after 3 h infusion of saline, a low or a high dose of rhIL-6 (Con, LoIL-6 and HiIL-6, respectively). Data are presented as means ±s.e.m. (n = 6). # Significantly different from Con (P < 0.05); $ Significantly different from LoIL-6 (P < 0.05).
There was a gradual decline (P < 0.05) in the rate of glucose appearance (Ra) and a concomitant fall (P < 0.05) in whole-body rate of glucose disappearance (Rd) in all trials, without any significant differences between the groups (Fig. 2A and B). As a consequence of the similar fall in both Ra and Rd for glucose in all trials, neither the metabolic clearance rate (MCR) of glucose (Fig. 2C) nor the arterial glucose concentration (Fig. 3A) was affected by time or treatment.
Figure 2. Effect of infusion of high and low doses of rhIL-6 on the rate of appearance (Ra) and disappearance (Rd) of 6,6-2H glucose, and on the metabolic clearance rate (MCR).

[6, 6-2H] glucose Ra (A), Rd (B) and MCR (C) before (Pre), during and after 3 h infusion of saline, a low or a high dose of rhIL-6. Data are presented as means ±s.e.m. (n = 6). § Time effect for Con, LoIL-6 and HiIL-6 (P < 0.05).
Figure 3. Effect of infusion of high and low doses of rhIL-6 on arterial glucose concentration and net leg-glucose uptake in the infusion leg.

Arterial glucose concentration (A) and net leg-glucose uptake in the infusion leg (B) before, during and after 3 h infusion of saline, a low or a high dose of rhIL-6. Data are presented as means ±s.e.m. (n = 6).
Since there were no differences between the infusion leg and the systemic leg during any trial for net leg-glucose uptake, muscle glycogen or GLUT 4 mRNA, the data are presented for the infusion leg only. Leg blood flow was not different when comparing the three trials (Table 1). In addition, net leg-glucose uptake, being the product of leg blood flow and femoral arterial-venous glucose concentration, was not different when comparing the trials (Fig. 3B). The muscle glycogen content averaged 370 ± 47 mmol glycosyl U (kg dry weight)−1 (n = 18) prior to infusion, and the content did not differ between groups or over time (Fig. 4). The three groups did not differ in the expression of GLUT 4 mRNA at any time point; however, the day after the infusion, GLUT 4 mRNA was lower (P < 0.05) in all groups (Fig. 5) compared with pre-infusion.
Table 1.
| Rest | 0.5 h | 1.0 h | 1.5 h | 2.0 h | 2.5 h | 3h | 1 h Post | 2 h Post | 3 h Post | |
|---|---|---|---|---|---|---|---|---|---|---|
| Con | 0.70 ± 0.10 | 0.67 ± 0.09 | 0.52 ± 0.12 | 0.65 ± 0.09 | 0.73 ± 0.10 | 0.62 ± 0.09 | 0.76 ± 0.10 | 0.63 ± 0.08 | 0.65 ± 0.07 | 0.76 ± 0.10 |
| L0IL6 | 0.70 ± 0.09 | 0.84 ± 0.14 | 0.71 ± 0.04 | 0.58 ± 0.04 | 0.56 ± 0.07 | 0.63 ± 0.04 | 0.89 ± 0.19 | 0.81 ± 0.09 | 0.90 ± 0.09 | 0.87 ± 0.14 |
| HiIL6 | 0.56 ± 0.06 | 0.76 ± 0.10 | 0.67 ± 0.13 | 0.59 ± 0.10 | 0.58 ± 0.07 | 0.54 ± 0.07 | 0.66 ± 0.07 | 0.69 ± 0.09 | 0.78 ± 0.08 | 0.79 ± 0.10 |
Limb blood flow (1 min−1) before (Rest), during and after 3 h infusion (Post) of saline, a low or a high dose of rhIL-6 (Con, L0IL6 or HiIL6, respectively). Data are presented as means ±s.e.m. (n = 6).
Figure 4. Effects of infusion of high and low doses of rhIL-6 on muscle glycogen content.

Muscle glycogen content in the infusion leg before, during and after 3 h infusion of either saline, a low or a high dose of rhIL-6. Data are presented as means +s.e.m. (n = 6).
Figure 5. Effects of infusion of high and low doses of rhIL-6 on muscle GLUT 4 mRNA.

Muscle GLUT 4 mRNA/28S rRNA before, during and after 3 h infusion of saline, a low or a high dose of rhIL-6. Data are presented as geometric means ±s.e.m. (n = 6). # Significantly different from pre-infusion for Con, LoIL-6 and HiIL-6 (P < 0.05).
The plasma hormone levels are reported in Fig. 6. Plasma insulin decreased (P < 0.05) over time, but there were no differences when comparing groups; nor was there a group × time interaction (Fig. 6A). Plasma glucagon levels were similar when comparing the three groups and this hormone was not affected by either time or treatment (Fig. 6B). There were no differences in plasma cortisol concentrations when comparing the three groups before infusion (Fig. 6C). Plasma cortisol concentrations did not change during Con, but increased (P < 0.05) during both HiIL-6 and LoIL-6. While concentrations of plasma cortisol declined after 2 h of infusion in LoIL-6, they remained elevated in HiIL-6 (P < 0.05) at 3 h of infusion. During both IL-6 trials, plasma cortisol levels returned to pre-infusion values after 3 h of recovery. Plasma noradrenaline was not affected by time or treatment when comparing trials (Fig. 7A). In contrast, plasma adrenaline markedly increased (P < 0.05) at the onset of infusion in HiIL-6 such that values after 60 min were greater (P < 0.05) compared with LoIL-6 and Con (Fig. 7B). It is of note that LoIL-6 did not affect plasma adrenaline concentrations.
Figure 6. Effects of infusion of high and low doses of rhIL-6 on plasma levels of insulin, glucagon and cortisol.

Plasma insulin (A), glucagon (B) and cortisol (C) before, during and after 3 h infusion of saline, a low or a high dose of rhIL-6. Data are presented as means ±s.e.m. (n = 6). § Time effect for Con, LoIL-6 and HiIL-6 (P < 0.05). * Significantly different from Pre (P < 0.05). # Significantly different from Con (P < 0.05). $ Significantly different from LoIL-6 (P < 0.05).
Figure 7. Effects of infusion of high and low doses of rhIL-6 on plasma levels of noradrenaline and adrenaline.
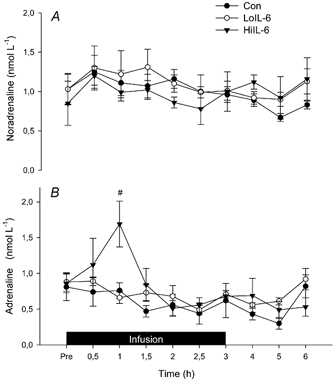
Plasma levels of noradrenaline (A) and adrenaline (B) before, during and after 3 h infusion of saline, a low or a high dose of rhIL-6. Data are presented as means ±s.e.m. (n = 6). # Significantly different from Con (P < 0.05).
DISCUSSION
Contrary to our hypothesis, the results from this study demonstrate, on the one hand, that rhIL-6 infusion does not increase whole-body glucose disposal or muscle glucose uptake. On the other hand, it is important to note that these results clearly demonstrate that elevated systemic IL-6 does not impair muscle glucose uptake or whole-body glucose disposal. Hence, even though circulating IL-6 is elevated in patients with type 2 diabetes mellitus, and has been implicated in insulin resistance and/or impaired glucose disposal (Tsigos et al. 1997), our data suggest that acutely elevating IL-6 does not impair muscle glucose uptake or whole-body glucose disposal in healthy humans.
We have shown previously that the release of IL-6 during prolonged exercise is related to intramuscular glycogen content (Keller et al. 2001; Steensberg et al. 2001). This led us (Pedersen et al. 2001) and others (Gleeson, 2000) to hypothesize that IL-6 was released by skeletal muscle when intramuscular glycogen content was low in order to provide a signal to the liver to increase endogenous glucose production (EGP). Despite the fact that EGP occurs rapidly at the onset of exercise, prior to any release of IL-6 from the skeletal muscle (Steensberg et al. 2001), this hypothesis was supported by previous studies in which it was found that rhIL-6 infusion increased either circulating glucose (Tsigos et al. 1997) or isotopic tracer-determined EGP (Stouthard et al. 1995). However, it must be noted that during these previous studies, the glucoregulatory hormones glucagon, adrenaline and noradrenaline were altered to some extent by rhIL-6 infusion. In contrast, the present study found that rhIL-6 infusion during LoIL-6 did not affect the circulating concentration of any of these hormones. The results from the present study demonstrate, therefore, that elevating IL-6 increases neither EGP (Fig. 2A) nor arterial glucose concentration (Fig. 3A) in healthy humans.
In the present study we hypothesized that IL-6 infusion would increase glucose uptake by the muscle. This hypothesis was based on previous studies that have examined the effect of IL-6 on specific cells in vitro. Stouthard et al. (1996) demonstrated that IL-6 increased both basal and insulin-stimulated glucose uptake in cultured 3T3-L1 adipocytes. These authors concluded that IL-6 acted by increasing intrinsic glucose transporter activity. In addition, Hardin et al. (2000) recently observed increased glucose transport in jejunal tissue incubated with IL-6 compared with controls. The authors concluded that IL-6 was able to increase the absorption of glucose in the gut, thereby increasing the plasma glucose levels. Recently it was shown that IL-6-knock-out mice developed impaired glucose disposal during an intravenous glucose tolerance test (Wallenius et al. 2002). Stouthard et al. (1995) also demonstrated that infusion of recombinant human IL-6 (rhIL-6) into renal cancer patients increased whole-body glucose disposal and subsequent oxidation compared with a control trial. Although one could argue that this was due to elevated glucagon increasing EGP, thereby resulting in an augmented whole-body glucose disposal, this appears unlikely since the metabolic clearance rate of glucose was higher in the rhIL-6 infusion trial. Hence, in this previous study (Stouthard et al. 1995) the relative hyperglycaemia was not responsible for the augmented glucose disposal. It must be noted that in this previous study, the rhIL-6 was infused at a rate even greater than the rate of HiIL-6 infusion in the present study. Furthermore, the subjects were cancer patients, and had increased energy expenditure during the IL-6 infusion, which in itself can increase glucose utilization. It is possible, therefore, that the different results in glucose disposal when comparing the previous with the present results are due to these methodological differences.
In the present study, we observed a small, but nonetheless significant decline in both glucose Ra and Rd throughout the experiment (Fig. 2). It should be noted that subjects arrived in the laboratory after an overnight fast and, therefore, this result is probably due to a progressive decline in hepatic glycogen concentration, in the absence of any carbohydrate ingestion throughout the experimental period. In addition, GLUT 4 mRNA was lower after 24 h in all trials when compared with pre-infusion. We have no definitive data to determine the reason for such an observation. However, apart from travelling to the laboratory on the morning of the experiments, subjects were bed rested for 20–24 h, which is known to decrease GLUT 4 expression (Tabata et al. 1999). It is also possible that slight muscle damage associated with the muscle biopsy procedure decreased GLUT 4 mRNA after 24 h, since muscle damage also decreases GLUT 4 protein expression (Asp et al. 1996). This latter suggestion appears less likely because the repeated biopsy procedure has no effect on HSP72 mRNA (Febbraio et al. 2002), which is a very sensitive index of cellular stress and trauma.
Our hypothesis that IL-6 increases glucose uptake was based on our previous observations that IL-6 expression and release in human muscle during contraction coincided with a concomitant increase in glucose uptake (Steensberg et al. 2001), while IL-6 release during graded exercise was positively correlated with leg-glucose uptake (Helge et al. 2002). In order to test our hypothesis, we chose to infuse rhIL-6 into the leg. Although we did not observe an enhanced leg-glucose uptake, our method cannot categorically rule out the possibility that IL-6, produced within the muscle, stimulates glucose uptake. Infused rhIL-6 could act in an alternative way to that which is produced endogenously by the muscle, if the muscle-derived IL-6 is either a different isoform or if IL-6 becomes active within the muscle only in a phosphorylated or glycosylated state. However, this seems unlikely because we have shown recently that infusion of rhIL-6 into the femoral artery of humans activates the stress protein HSP72 (Febbraio et al. 2002). These recent data demonstrate that the form of IL-6 infused in the present study is biologically active in human skeletal muscle.
Recently Lyngsøet al. (2002) demonstrated that acute rhIL-6 infusion markedly increased circulating FFA concentration. According to the classic glucose-fatty acid cycle of Randle and co-workers (1963), such an increase would result in transient insulin resistance. Indeed, it has been demonstrated that acute increases in plasma FFA decrease insulin-stimulated glucose uptake in rats (Kim et al. 1996). However, our results demonstrate clearly that acute rhIL-6 infusion does not result in transient insulin resistance.
Since we hypothesized that IL-6 would increase glucose uptake, we expected glycogen content to be higher following IL-6 infusion as the increase in glucose uptake would result in enhanced glycogen storage. Despite the markedly elevated levels of IL-6 in the infusion leg during HiIL-6 and LoIL-6, intramuscular glycogen content was not affected. The fact that muscle glycogenolysis was not increased in HiIL-6 may, on the surface, appear anomalous with respect to the marked increase in adrenaline, since adrenaline is know to increase muscle glycogenolysis (Jansson et al. 1986). It must be noted, however, that adrenaline appears to exert its glycogenolytic effect in active rather that resting skeletal muscle. During contraction, adrenaline converts glycogen phosphorylase (Phos), the enzyme responsible for glycogenolysis from the inactive b form to the active a form, and the concomitant increase in inorganic phosphate (Pi) provides substrate to keep Phos in the a form. However, at rest there is insufficient Pi to keep Phos in the active form and, even in the presence of adrenaline, it rapidly converts back to the inactive form (Ren & Hultman, 1989). Hence, the fact that glycogenolysis was not increased in HiIL-6 is consistent with this rationale.
Recently, Metzger et al. (2001) found that mice bearing IL-6-secreting tumours had reduced body fat. In addition, Wallenius et al. (2002) demonstrated that IL-6-deficient mice become markedly glucose intolerant and obese, while treatment of these mice with IL-6 partially attenuated these perturbations. They suggested that IL-6 could be used as a therapeutic drug in the treatment of obesity-related illnesses. It was noteable that, in the present study, rhIL-6 infusion during LoIL-6 did not result in adverse side effects and there were no clinical symptoms reported by the subjects or changes in sympathetic or pancreatic hormones. In addition, rhIL-6 infusion did not increase EGP or arterial glucose concentration, demonstrating that therapeutic IL-6 treatment in obese and/or insulin-resistant patients would not result in hyperglycaemia, thereby reducing the likelihood of the adverse complications associated with glucose toxicity.
In conclusion, we have shown that acute IL-6 administration does not impair whole-body glucose disposal or net leg-glucose uptake, or increase endogenous glucose production during rest. Hence, a transient increase in systemic concentration of IL-6 does not result in impaired glucose homeostasis.
Acknowledgments
The authors wish to thank the subjects who participated in this study for their extraordinary effort. In addition, the technical assistance of Ruth Rousing, Carsten Nielsen, Birgitte Jessen, Hanne Villumsen, Kristina Møller, Kirsten Møller, Natalie Hiscock, Ann-Christine Henriksen and Vigdis Christie is greatly acknowledged. This study was supported by the Danish National Research Foundation (504–14), NovoNordisk Foundation and The Danish Medical Research Council (22-01-0019). Takuya Osada is a Visiting Fellow from the Department of Preventive Medicine and Public Health, Tokyo Medical University, Tokyo, Japan.
references
- Asp S, Daugaard JR, Kristiansen S, Kiens B, Richter EA. Eccentric exercise decreases maximal insulin action in humans: muscle and systemic effects. J Physiol. 1996;494:891–898. doi: 10.1113/jphysiol.1996.sp021541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrand E, Saltin B. Effect of muscle glycogen on glucose, lactate and amino acid metabolism during exercise and recovery in human subjects. J Physiol. 1999;514:293–302. doi: 10.1111/j.1469-7793.1999.293af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Fischer CP, Keller C, Hiscock N, Pedersen BK. IL-6 activates HSP72 gene expression in human skeletal muscle. Biochem Biophys Res Comm. 2002;296:1264–1266. doi: 10.1016/s0006-291x(02)02079-x. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Muscle derived inerleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Interleukins and exercise. J Physiol. 2000;529:1. doi: 10.1111/j.1469-7793.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J, Kroeker K, Chung B, Gall DG. Effect of proinflammatory interleukins on jejunal nutrient transport. Gut. 2000;47:184–191. doi: 10.1136/gut.47.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helge JW, Stallnecht B, Pedersen BK, Galbo H, Kiens B, Richter EA. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol. 2003;546:299–309. doi: 10.1113/jphysiol.2002.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. Intern Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- Jansson E, Hjemdahl P, Kaijser L. Epinephrine induced changes in muscle carbohydrate metabolism during exercise in male subjects. J Appl Physiol. 1986;60:1466–1470. doi: 10.1152/jappl.1986.60.5.1466. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Pedersen BK, Neufer D. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle - influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- Kim JK, Wi JK, Youn JH. Plasma free fatty acids decrease insulin-stimulated skeletal muscle glucose uptake by suppressing glycolysis in conscious rats. Diabetes. 1996;45:446–453. doi: 10.2337/diab.45.4.446. [DOI] [PubMed] [Google Scholar]
- Langberg H, Olesen JL, Gemmer C, Kjær M. Substantial elevation of interleukin-6 concentration in peritendonous, but not muscle, following prolonged exercise. J Physiol. 2002;542:985–990. doi: 10.1113/jphysiol.2002.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngsø D, Simonsen L, Bülow J. Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. J Physiol. 2002;543:379–386. doi: 10.1113/jphysiol.2002.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger S, Hassin T, Barash V, Pappo O, Chajek-Shaul T. Reduced body fat and increased hepatic lipid synthesis in mice bearing interleukin-6-secreting tumor. Am J Physiol. 2001;281:E957–965. doi: 10.1152/ajpendo.2001.281.5.E957. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B, Pedersen BK, Møller K, Secher NH. Interleukin-6 release from human brain during exercise. J Physiol. 2002;542:991–995. doi: 10.1113/jphysiol.2002.022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536:329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rådegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle, its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Ren J-M, Hultman E. Regulation of glycogenolysis in human skeletal muscle. J Appl Physiol. 1989;67:2243–2248. doi: 10.1152/jappl.1989.67.6.2243. [DOI] [PubMed] [Google Scholar]
- Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol. 2001;533:585–591. doi: 10.1111/j.1469-7793.2001.0585a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Febbraio MA, Osada T, Van Hall G, Saltin B, Pedersen BK. Low glycogen content increases interleukin-6 production in contracting human skeletal muscle. J Physiol. 2001;537:633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Keller C, van Hall G, Osada T, Schjerling P, Pedersen BK, Saltin B, Febbraio MA. Muscle glycogen content and glucose uptake during exercise in humans: influence of prior exercise and dietary manipulation. J Physiol. 2002;541:273–281. doi: 10.1113/jphysiol.2001.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise induced increase in plasma interleukin-6. J Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthard JM, Romijn JA, Van Der Poll T, Endert E, Klein S, Bakker PJ, Veenhof CH, Sauerwein HP. Endocrinologic and metabolic effects of interleukin-6 in humans. Am J Physiol. 1995;268:E813–819. doi: 10.1152/ajpendo.1995.268.5.E813. [DOI] [PubMed] [Google Scholar]
- Stouthard JM, Oude Elferink RP, Sauerwein HP. Interleukin-6 enhances glucose transport in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1996;18:241–245. doi: 10.1006/bbrc.1996.0389. [DOI] [PubMed] [Google Scholar]
- Tabata I, Suzuki Y, Fukunaga T, Yokozeki T, Akimi H, Funato K. Resistance training affects GLUT-4 content in skeletal muscle of humans after 19 days of head-down bed rest. J Appl Physiol. 1999;86:909–914. doi: 10.1152/jappl.1999.86.3.909. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab. 1997;82:4167–4170. doi: 10.1210/jcem.82.12.4422. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudlin M, Carlsten H, Dickson SL, Ohlsson C, Jansson J-O. Interleukin-6-deficient mice develop mature-onset obesity. Nature Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]


