Abstract
To examine whether glucose ingestion during exercise affects the release of interleukin-6 (IL-6) from the contracting limb, seven men performed 120 min of semi-recumbent cycling on two occasions while ingesting either 250 ml of a 6.4 % carbohydrate (GLU trial) or sweet placebo (CON trial) beverage at the onset of, and at 15 min intervals throughout, exercise. Muscle biopsies obtained before and immediately after exercise were analysed for glycogen and IL-6 mRNA expression. Blood samples were simultaneously obtained from a brachial artery and a femoral vein prior to and during exercise and leg blood flow was measured by thermodilution in the femoral vein. Net leg IL-6 release, and net leg glucose and free fatty acid (FFA) uptake, were calculated from these measurements. The arterial IL-6 concentration was lower (P < 0.05) after 120 min of exercise in GLU, but neither intramuscular glycogen nor IL-6 mRNA were different when comparing GLU with CON. However, net leg IL-6 release was attenuated (P < 0.05) in GLU compared with CON. This corresponded with an enhanced (P < 0.05) glucose uptake and a reduced (P < 0.05) FFA uptake in GLU. These results demonstrate that glucose ingestion during exercise attenuates leg IL-6 release but does not decrease intramuscular expression of IL-6 mRNA.
The cytokine interleukin-6 (IL-6) increases markedly in the circulation during exercise. Although the peritendon (Langberg et al. 2002) and brain (Nybo et al. 2002) release small amounts of IL-6 into the circulation during exercise, the major source of the increase in circulating IL-6 is the contracting skeletal muscle (Febbraio & Pedersen, 2002). Specifically, contraction rapidly increases intramuscular mRNA expression (Ostrowski et al. 1998; Keller et al. 2001; Starkie et al. 2001a; Steensberg et al. 2001) and the nuclear transcriptional activity of IL-6 (Keller et al. 2001), leading to marked IL-6 protein release from the contracting limb (Steensberg et al. 2000, 2001). Interestingly, mRNA expression (Steensberg et al. 2001; Keller et al. 2001), the nuclear transcriptional rate (Keller et al. 2001) and leg protein release (Steensberg et al. 2001) of IL-6 are augmented during exercise in the presence of a lower pre-exercise muscle glycogen concentration, leading to the suggestion that one biological role of exercise-induced IL-6 release is to regulate substrate mobilization and subsequent oxidation (Febbraio & Pedersen, 2002). This hypothesis is supported by the observation that preventing a fall in plasma glucose by carbohydrate ingestion reduces circulating plasma IL-6 during exercise (Nehlsen-Cannarella et al. 1997; Nieman et al. 1998; Starkie et al. 2001a; Bishop et al. 2002; Nieman et al. 2003). These studies did not, however, measure tissue release of IL-6 and, therefore, little is known regarding the mechanism by which glucose ingestion attenuates the plasma IL-6 response.
Of note, two previous studies have examined the effect of glucose ingestion on contracting skeletal muscle glycogenolysis and IL-6 mRNA (Starkie et al. 2001a; Nieman et al. 2003). In both of these studies carbohydrate ingestion did not affect muscle glycogenolytic rate, but while the former observed very similar fold increases in IL-6 mRNA in the skeletal muscle, the latter observed a tendency for the contraction-induced IL-6 mRNA to decrease with glucose ingestion. This latter finding was surprising, given the fact that IL-6 gene transcription during exercise appears to be mediated, in part, by glycogen content. Clearly, more research is required to examine the effects of glucose ingestion on the skeletal muscle IL-6 response to exercise and in the present study we aimed to determine the effect of glucose ingestion on IL-6 mRNA expression within and protein release from skeletal muscle. We hypothesized that whilst glucose ingestion would blunt the plasma IL-6 response, it would have no effect on the response in skeletal muscle.
METHODS
Subjects
Seven men (22.1 ± 3.8 years old; 182.1 ± 5.5 cm tall; 81.0 ± 12.7 kg body weight; maximal oxygen uptake (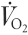 ,max) 3.88 ± 0.34 l min−1; means ±s.d.) participated in the study, which was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark, and performed according to the Declaration of Helsinki. Subjects were informed about the possible risks and discomfort involved before their written consent was obtained.
,max) 3.88 ± 0.34 l min−1; means ±s.d.) participated in the study, which was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark, and performed according to the Declaration of Helsinki. Subjects were informed about the possible risks and discomfort involved before their written consent was obtained.
Preliminary testing
Each subject underwent preliminary medical screening and was exempt from the study if he presented contra-indications. Following the medical screening, each subject underwent a maximal oxygen uptake (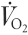 ,max) test on a semi-recumbent cycle ergometer. A workload was calculated that would elicit ∼65 % of each individual's
,max) test on a semi-recumbent cycle ergometer. A workload was calculated that would elicit ∼65 % of each individual's 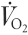 ,max. Forty-eight hours prior to the experimental trials, subjects reported to the laboratory and completed 45 min of upright cycling exercise at a workload corresponding to 65 % of maximal heart rate. Thereafter, subjects were provided with food packages that they consumed for the next 2 days (15.6 MJ per day, approximately 70 % carbohydrate, 15 % protein and 15 % fat). During this period the subjects were asked to adhere to the diet and to refrain from strenuous exercise and the intake of alcohol, tobacco and caffeine. This regime was adopted to minimize any differences in pre-exercise intramuscular glycogen levels in each subject when comparing trials.
,max. Forty-eight hours prior to the experimental trials, subjects reported to the laboratory and completed 45 min of upright cycling exercise at a workload corresponding to 65 % of maximal heart rate. Thereafter, subjects were provided with food packages that they consumed for the next 2 days (15.6 MJ per day, approximately 70 % carbohydrate, 15 % protein and 15 % fat). During this period the subjects were asked to adhere to the diet and to refrain from strenuous exercise and the intake of alcohol, tobacco and caffeine. This regime was adopted to minimize any differences in pre-exercise intramuscular glycogen levels in each subject when comparing trials.
Experimental procedures
Subjects participated in two experimental trials separated by at least 10 days and conducted in random order. During each trial the subjects exercised on a semi-recumbent cycle ergometer for 120 min. They commenced exercise for 5 min at 50 % 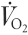 ,max and subsequently cycled for 115 min at ∼65 %
,max and subsequently cycled for 115 min at ∼65 % 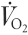 ,max. Each trial was identical except that in one trial (GLU) subjects ingested 250 ml of a 6.4 % carbohydrate beverage (Lucozade Sport; GlaxoSmithKline UK) at the onset of, and at 15 min intervals throughout, exercise whereas in the other (CON) they consumed the same quantity of an artificially flavoured placebo. On the day of each experiment the subjects reported to the laboratory at 07.30 h after a 12–14 h overnight fast. They voided, changed into appropriate exercise attire and rested supine for 10 min. A catheter was then placed in the left brachial artery (1.0 mm i.d.; 20 gauge) and a second catheter (7 diameter; Cook, Denmark) was inserted into the left femoral vein ∼1–2 cm distal to the inguinal ligament (Febbraio et al. 2002). Leg blood flow (LBF) was measured at rest and at 30 min intervals during exercise using the constant infusion thermodilution technique, as described previously (Anderson & Saltin, 1985; Febbraio et al. 2002).). Briefly, venous blood and infusate temperatures were measured continuously during saline infusion (45 s, 20 ml min−1 for resting measurements; 20 s, 100 ml min−1 for exercising measurements; Harvard pump, Harvard Apparatus, Millis, MA, USA) via thermistors connected to a custom-made electronic box. The electronic box was interfaced to a computer (Macintosh Performa) using a data acquisition system (MacLab 8:s, ADInstruments, Sydney, Australia). Venous blood temperature was measured with a thermistor (model 94-030-2.5Fr T. D. Probe, Edwards Edslab, Baxter, Irvine, CA, USA) inserted, via the femoral venous catheter, in the proximal direction. The thermistor probe was positioned 9 cm beyond the tip of the 8 cm catheter, which was perforated with four side-holes to facilitate infusate dispersion. Infusate temperature (2–3 °C) was measured with a flow-through housing (model 93–505, Edslab) at the site where the infusate entered the venous catheter. Infusate temperature was corrected for the 0.6 °C increase which occurs as the infusate travels from the measuring point to the tip of the catheter (Anderson & Saltin, 1985). At rest, infusate temperature was corrected by 1.0 °C. LBF (expressed in ml min−1) was calculated according to the formula derived from a heat balance equation (Anderson & Saltin, 1985).
,max. Each trial was identical except that in one trial (GLU) subjects ingested 250 ml of a 6.4 % carbohydrate beverage (Lucozade Sport; GlaxoSmithKline UK) at the onset of, and at 15 min intervals throughout, exercise whereas in the other (CON) they consumed the same quantity of an artificially flavoured placebo. On the day of each experiment the subjects reported to the laboratory at 07.30 h after a 12–14 h overnight fast. They voided, changed into appropriate exercise attire and rested supine for 10 min. A catheter was then placed in the left brachial artery (1.0 mm i.d.; 20 gauge) and a second catheter (7 diameter; Cook, Denmark) was inserted into the left femoral vein ∼1–2 cm distal to the inguinal ligament (Febbraio et al. 2002). Leg blood flow (LBF) was measured at rest and at 30 min intervals during exercise using the constant infusion thermodilution technique, as described previously (Anderson & Saltin, 1985; Febbraio et al. 2002).). Briefly, venous blood and infusate temperatures were measured continuously during saline infusion (45 s, 20 ml min−1 for resting measurements; 20 s, 100 ml min−1 for exercising measurements; Harvard pump, Harvard Apparatus, Millis, MA, USA) via thermistors connected to a custom-made electronic box. The electronic box was interfaced to a computer (Macintosh Performa) using a data acquisition system (MacLab 8:s, ADInstruments, Sydney, Australia). Venous blood temperature was measured with a thermistor (model 94-030-2.5Fr T. D. Probe, Edwards Edslab, Baxter, Irvine, CA, USA) inserted, via the femoral venous catheter, in the proximal direction. The thermistor probe was positioned 9 cm beyond the tip of the 8 cm catheter, which was perforated with four side-holes to facilitate infusate dispersion. Infusate temperature (2–3 °C) was measured with a flow-through housing (model 93–505, Edslab) at the site where the infusate entered the venous catheter. Infusate temperature was corrected for the 0.6 °C increase which occurs as the infusate travels from the measuring point to the tip of the catheter (Anderson & Saltin, 1985). At rest, infusate temperature was corrected by 1.0 °C. LBF (expressed in ml min−1) was calculated according to the formula derived from a heat balance equation (Anderson & Saltin, 1985).
Immediately prior to exercise and at 30, 60, 90 and 120 min during exercise, blood samples (10 ml) were simultaneously collected from the brachial artery and the femoral vein for the measurement of IL-6 by a highly sensitive enzyme-linked immunosorbent assay. Glucose and free fatty acids (FFA) were determined using automated analyses (Cobas Fara, Roche, France) (Steensberg et al. 2001, 2002). Blood samples were also analysed for haematocrit (Hct) using an automated analyser (Radiometer, OSM-2 Hemoximeter, Copenhagen Denmark). Immediately prior to sampling, 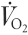 , respiratory exchange ratio (RER) and heart rate (HR) were recorded (Febbraio et al. 2002). Muscle biopsy samples were obtained from the vastus lateralis muscle prior to and immediately following exercise and analysed for glycogen using enzymatic analyses with fluorometric detection and IL-6 mRNA using real-time PCR (Steensberg et al. 2001).
, respiratory exchange ratio (RER) and heart rate (HR) were recorded (Febbraio et al. 2002). Muscle biopsy samples were obtained from the vastus lateralis muscle prior to and immediately following exercise and analysed for glycogen using enzymatic analyses with fluorometric detection and IL-6 mRNA using real-time PCR (Steensberg et al. 2001).
Calculations and statistics
Leg plasma flow (LPF) was calculated from the LBF and Hct measures. Net leg IL-6 release, and net leg glucose uptake were calculated by multiplying the femoral vein-arterial [IL-6] and [glucose] by the net LBF. Similarly, the net FFA uptake was calculated by multiplying femoral vein-arterial [FFA] by the net LPF. Comparative data are expressed as means ±s.e.m. A two-way (trial × time) analysis of variance (ANOVA) with repeated measures on the time factor was used to compute the statistics (Statistica, Tulsa, OK, USA), with significance accepted as P < 0.05. If analyses revealed a significant interaction, a Newman-Keuls post hoc test was used to locate specific differences.
RESULTS
Although 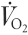 increased (P < 0.05) when comparing exercise with rest in both trials, there were no differences in this measure when comparing trials (Table 1). Both RER and HR increased (P < 0.05) during exercise compared with rest in both trials. In addition, both RER and HR were higher (P < 0.05) in GLU after 120 min of exercise than in CON (Table 1). While the difference in RER was expected, due to increased glucose availability resulting in augmented carbohydrate oxidation, the reason for the small increase in HR was inexplicable in light of the similar
increased (P < 0.05) when comparing exercise with rest in both trials, there were no differences in this measure when comparing trials (Table 1). Both RER and HR increased (P < 0.05) during exercise compared with rest in both trials. In addition, both RER and HR were higher (P < 0.05) in GLU after 120 min of exercise than in CON (Table 1). While the difference in RER was expected, due to increased glucose availability resulting in augmented carbohydrate oxidation, the reason for the small increase in HR was inexplicable in light of the similar 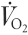 at this time.
at this time.
Table 1.
Oxygen consumption ( ), respiratory exchange ratio (RER) and heart rate (HR), before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake with (GLU) or without (CON) the ingestion of glucose throughout exercise
), respiratory exchange ratio (RER) and heart rate (HR), before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake with (GLU) or without (CON) the ingestion of glucose throughout exercise
| Parameter | Trial | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|---|
 (l min−1) (l min−1) |
CON | 0.27 ± 0.06 | 2.48 ± 0.17 | 2.45 ± 0.15 | 2.50 ± 0.15 | 2.44 ± 0.15 |
| GLU | 0.31 ± 0.07 | 2.39 ± 0.14 | 2.33 ± 0.13 | 2.46 ± 0.14 | 2.48 ± 0.14 | |
| RER | CON | 0.81 ± 0.07 | 0.92 ± 0.05 | 0.90 ± 0.05 | 0.88 ± 0.05 | 0.85 ± 0.05 |
| GLU | 0.78 ± 0.05 | 0.91 ± 0.04 | 0.91 ± 0.04 | 0.91 ± 0.04 | 0.89 ± 0.05* | |
| HR (beats min−1) | CON | 60 ± 1 | 144 ± 1 | 148 ± 1 | 149 ± 1 | 150 ± 1 |
| GLU | 68 ± 1 | 146 ± 1 | 148 ± 1 | 157 ± 1 | 159 ± 1* |
P < 0.05 when comparing trials. Data expressed as means ±s.e.m. (n = 7).
LBF increased (P < 0.05) from 0.54 ± 0.07 and 0.56 ± 0.06 l min−1 at rest to an average of 6.12 ± 0.52 and 6.14 ± 0.38 l min−1 during exercise in CON and GLU, respectively. LPF was ∼43 % lower than LBF but followed a similar pattern in that there were no differences when comparing trials (data not shown).
There were no differences in arterial plasma [IL-6] when comparing trials at rest. Whilst the arterial plasma [IL-6] increased (P < 0.05) in both trials, it rose to a greater extent in CON such that the values at 120 min were higher (P < 0.05) in CON than in GLU (13.8 ± 2.7 vs. 7.4 ± 0.8 ng l−1 for CON and GLU, respectively). Net leg IL-6 was released (P < 0.05) during exercise in CON, but not in GLU. As a result net leg IL-6 release was higher (P < 0.05) in CON than in GLU throughout the last 60 min of exercise (Fig. 1).
Figure 1. Arterial plasma [IL-6] and leg IL-6 release before and during semi-recumbent cycling with or without the ingestion of glucose.

Arterial plasma [IL-6] (top) and leg IL-6 release (bottom) before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake with (□) or without (•) the ingestion of glucose throughout exercise.* Significant difference (P < 0.05) between trials. Data expressed as means ±s.e.m. (n = 7).
Arterial plasma [glucose] was similar when comparing trials at rest. However, throughout exercise arterial plasma [glucose] was higher (P < 0.05) in GLU than in CON (Fig. 2). Whilst leg glucose uptake increased (P < 0.05) in both trials, it increased to a greater extent in GLU such that it was higher (P < 0.05) than in CON throughout the last 60 min of exercise (Fig. 2).
Figure 2. Arterial plasma [glucose] and leg glucose uptake before and during semi-recumbent cycling with or without the ingestion of glucose.
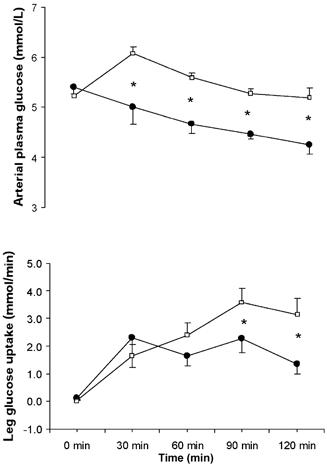
Arterial plasma [glucose ](top) and leg glucose uptake (bottom) before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake with (□) or without (•) the ingestion of glucose throughout exercise.* Significant difference (P < 0.05) between trials. Data expressed as means ±s.e.m. (n = 7).
Arterial plasma [FFA] was similar when comparing trials at rest. However, the arterial plasma [FFA] increased (P < 0.05) after 30 min of exercise in CON, but remained stable in GLU. As a consequence, arterial plasma [FFA] was higher (P < 0.05) during the last 60 min of exercise in CON than in GLU (Fig. 3). While net leg FFA uptake increased (P < 0.05) in both trials, it increased to a greater extent in CON such that it was higher (P < 0.05) when compared with GLU throughout the latter 60 min of exercise (Fig. 3).
Figure 3. Arterial plasma [FFA] and leg FFA uptake before and during semi-recumbent cycling with or without the ingestion of glucose.
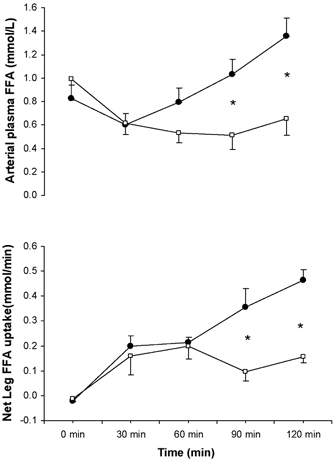
Arterial plasma [FFA] (top) and leg FFA uptake (bottom) before (0 min) and during 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake with (□) or without (•) the ingestion of glucose throughout exercise.* Significant difference (P < 0.05) between trials. Data expressed as means ±s.e.m. (n = 7).
Muscle glycogen content was not different when comparing trials at rest. Whilst the glycogen content decreased (P < 0.05) in both trials, the rate of glycogenolysis was identical (Fig. 4). There was a ∼20-fold increase in IL-6 mRNA with exercise and the fold increase was not different when comparing CON with GLU (Fig. 4).
Figure 4. Intramuscular muscle glycogen content and IL-6 mRNA during semi-recumbent cycling with or without the ingestion of glucose.
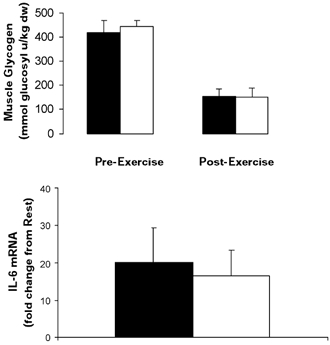
Intramuscular muscle glycogen (top) and IL-6 mRNA (bottom) contents before and after 120 min of semi-recumbent cycling at 62 ± 2 % of maximal oxygen uptake with (□) or without (▪) the ingestion of glucose throughout exercise. Data expressed as means ±s.e.m. (n = 7).
Discussion
The results from this study demonstrate that glucose ingestion attenuates IL-6 release from the contracting limb and hence provide a mechanism for the observation that glucose ingestion attenuates the plasma IL-6 response during exercise. Furthermore, since the exercise-induced increase in IL-6 mRNA was not affected by glucose ingestion during exercise, these data raise the possibility that the process of IL-6 release from skeletal muscle may be regulated by substrate availability and/or flux across the contracting skeletal muscle.
These data are consistent with the observation that glucose ingestion blunts the plasma IL-6 response (Nehlsen-Cannarella et al. 1997; Nieman et al. 1998; Bishop et al. 2002). In addition, the present data are consistent with the previous finding (Starkie et al. 2001a) that glucose ingestion blunted circulating IL-6 in the presence of an unaltered increase in contracting muscle IL-6 mRNA. In both the previous (Starkie et al. 2001a) and the present study, muscle glycogen content was not different when comparing trials and it is well known that the rate of muscle glycogenolysis is unaffected by glucose ingestion during cycling exercise (Jeukendrup & Jentjens, 2000). The fold increase in IL-6 mRNA (Keller et al. 2001; Steensberg et al. 2001) and the transcriptional rate of IL-6 from the nuclei of contracting skeletal muscle fibres (Keller et al. 2001) are influenced by glycogen content. Even though there was an increase in skeletal muscle glucose uptake during exercise, this resulted in increased carbohydrate oxidation, as indicated by the higher RER (Table 1), rather than a reduced rate of glycogenolysis. Hence, the present data support the hypothesis that IL-6 mRNA is influenced by glycogen content, but also provide evidence that IL-6 gene expression is unaffected by enhanced glucose uptake or oxidation in the contracting skeletal muscle. The data are, however, in partial contrast to a recent observation by Nieman and colleagues (2003). In this recent study, the authors also demonstrated that carbohydrate ingestion attenuated the plasma IL-6 response, but did not affect the rate of muscle glycogenolysis during a 3 h treadmill run. However, the authors reported a tendency for IL-6 mRNA also to be reduced. In the present study, we saw no such tendency and our data support our previous study (Starkie et al. 2001a). It must be noted however, that in the present study our subjects cycled for 2 h while in our previous study they either ran or cycled for 60 min. In contrast, in the study by Nieman et al. (2003) subjects exercised for ∼3 h. Hence, the disparity in exercise duration when comparing the present study with that recently reported (Nieman et al. 2003) may account for the observed differences in IL-6 mRNA.
The previous observation of a decreased circulating IL-6 content in the presence of an unaltered increase in contracting muscle IL-6 mRNA (Starkie et al. 2001a) led to the possibility that other tissues could be producing IL-6 during exercise and that this production was attenuated by glucose ingestion. Although the contracting limb accounts for much of the circulating IL-6 during exercise (Steensberg et al. 2000), evidence is indeed emerging that other tissues also release IL-6 during exercise. Neither the blood mononuclear cells (Starkie et al. 2001b) nor adipose tissue (Lyngsøet al. 2002) produce IL-6 during exercise, but it has recently been demonstrated that the peritendon (Langberg et al. 2002) and the brain (Nybo et al. 2002) can release IL-6 during exercise. In the present study, tissues other than those contained in the contracting limb may have produced IL-6 at different rates during exercise in each trial because a small increase in circulating IL-6 in GLU was observed in the presence of totally blunted net leg IL-6 release (Fig. 1). Whether glucose ingestion affects IL-6 release by the brain or peritendon has not been experimentally investigated. We have also recently demonstrated that IL-6 is cleared by the hepatosplanchnic viscera during exercise (Febbraio et al. 2003). With these data in mind, we cannot rule out the possibility that glucose ingestion enhanced the hepatosplanchnic clearance of IL-6. However, we have performed preliminary experiments in four individuals and these preliminary data suggest that glucose ingestion does not affect the hepatosplanchnic clearance of IL-6 (M. A. Febbraio, A. Steensberg, C. Keller, R. L. Starkie, H. B. Nielsen, P. Krustrup, P. Ott, N. H. Secher & B. K. Pedersen, unpublished observations).
Given that the exercise-induced IL-6 mRNA in contracting skeletal muscle was not influenced by glucose ingestion during exercise, it was unexpected that IL-6 release by the limb was attenuated by glucose ingestion (Fig. 1). This raises the possibility that IL-6 can undergo post-transcriptional modification, such that the rate of protein synthesis (the rate of translation of IL-6 mRNA) in the skeletal muscle is reduced by glucose feeding and/or that IL-6 release is transporter mediated and that the transporter mechanism is regulated by substrate availability. Due to technical difficulties, we could not measure IL-6 protein accumulation in the skeletal muscle. However, even if this were possible, the fact that IL-6 is released by skeletal muscle would make interpretation of the data difficult. Such a measure of IL-6 protein content would not allow us to determine the kinetics of protein synthesis with concomitant IL-6 protein release. Of note was the observation that both the arterial glucose concentration and leg glucose uptake were higher (Fig. 2) while circulating [FFA] and leg FFA uptake were lower (Fig. 3) in GLU than in CON. We (Febbraio & Pedersen, 2002) and others (Papanicolaou & Vgontzas, 2000) have hypothesized that IL-6 is released from tissues to act in an endocrine manner. It has recently been shown that recombinant human IL-6 infused into resting humans to levels that mimic plasma IL-6 concentration during vigorous, prolonged exercise results in marked lipolysis and fat oxidation in the absence of any changes in key lipolytic hormones (van Hall et al. 2003). We suggest therefore that during exercise IL-6 is released from the contracting limb as an ‘endocrine-like molecule’ signalling adipose tissue to increase lipolysis for subsequent oxidation. Hence, it is plausible to suggest that, in the present study, the increased glucose availability and leg glucose uptake diminished the requirement for FFA as a substrate, subsequently down-regulating IL-6 protein release from the contracting muscle. Such a suggestion is speculative, however, and warrants further investigation.
In summary, glucose ingestion during exercise attenuates leg IL-6 release but does not decrease intramuscular expression of IL-6 mRNA. The data suggest that IL-6 release by the contracting muscles during exercise is regulated by substrate availability.
Acknowledgments
We would like to thank the subjects for their extraordinary effort in this demanding study. We would also like to thank Ruth Rousing, Peter Nissen, Nina Schjerling, Ellen Dawson, Natalie Hiscock, Kristina Møller and Hanne Willumsen for their excellent technical assistance. This study was supported by grants from the Danish National Research Foundation (504–14) and GlaxoSmithKline.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NC, Gleeson M, Nicholas CW, Ali A. Influence of carbohydrate supplementation on plasma cytokine and neutrophil degranulation response to high intensity intermittent exercise. Int J Sports Nutr Exerc Metab. 2002;12:145–156. doi: 10.1123/ijsnem.12.2.145. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544:957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Hepatosplanchnic clearance of interleukin-6 in humans. Am J Physiol. 2003 doi: 10.1152/ajpendo.00134.2003. in the Press. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Muscle derived interleukin-6, mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Jentjens R. Oxidation of carbohydrate feedings during prolonged exercise: current thoughts, guidelines and directions for future research. Sports Med. 2000;29:407–424. doi: 10.2165/00007256-200029060-00004. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- Langberg H, Olesen JL, Gemmer C, Kjær M. Substantial elevation of interleukin-6 concentration in peritendonous, but not muscle, following prolonged exercise. J Physiol. 2002;542:985–990. doi: 10.1113/jphysiol.2002.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngsø D, Simonsen L, Bülow J. Interleukin-6 production in human subcutaneous abdominal adipose tissue: effect of exercise. J Physiol. 2002;543:1033–1046. doi: 10.1113/jphysiol.2002.019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, Bailey EM, Warren BJ, Utter A, Davis JM. Carbohydrate and the cytokine response to 2. 5 h of running. J Appl Physiol. 1997;82:1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol. 2003;94:1917–1925. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, Williams F, Butterworth DE. Influence of mode and carbohydrate on the cytokine response to heavy exertion. Med Sci Sports Exerc. 1998;30:671–678. doi: 10.1097/00005768-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B, Pedersen BK, Møller K, Secher NH. Interleukin-6 release from human brain during exercise. J Physiol. 2002;542:991–995. doi: 10.1113/jphysiol.2002.022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K, Rhode T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou DA, Vgontzas AN. Interleukin-6: the endocrine cytokine. J Clin Endocrinol Metab. 2000;85:1331–1333. doi: 10.1210/jcem.85.3.6582. [DOI] [PubMed] [Google Scholar]
- Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol. 2001a;533:585–591. doi: 10.1111/j.1469-7793.2001.0585a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA. Circulating monocytes are not the source of the elevations in plasma Il-6 and TNF-α levels after prolonged running. Am J Physiol Circ Physiol. 2001b;280:C769–774. doi: 10.1152/ajpcell.2001.280.4.C769. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537:633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Keller C, van Hall G, Osada T, Schjerling P, Pedersen BK, Saltin B, Febbraio MA. Muscle glycogen content and glucose uptake during exercise in humans: influence of prior exercise and dietary manipulation. J Physiol. 2002;541:273–281. doi: 10.1113/jphysiol.2001.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Møller K, Saltin B, Febbraio MA. Pedersen BK. Interleukin-6 stimulated lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003 doi: 10.1210/jc.2002-021687. (in the Press) [DOI] [PubMed] [Google Scholar]


