Abstract
The existence of cells storing and secreting two different anterior pituitary (AP) hormones (polyhormonal cells) or responding to several hypothalamic releasing hormones (HRHs) (multiresponsive cells) has been reported previously. These multifunctional cells could be involved in paradoxical secretion (AP hormone secretion evoked by a non-corresponding HRH) and transdifferentiation (phenotypic switch between mature cell types without cell division). Despite their putative physiological relevance, a comprehensive characterization of multifunctional AP cells is lacking. Here we combine calcium imaging (to assess responses to the four HRHs) and multiple sequential immunoassay of the six AP hormones in the same individual cells to perform a complete phenotypic characterization of mouse AP cells. Polyhormonal and multiresponsive cells were identified within all five AP cell types. They were scarce in the more abundant cell types, somatotropes and lactotropes, but quite frequent in corticotropes and gonadotropes. Cells with mixed phenotypes were the rule rather than the exception in thyrotropes, where 56–83 % of the cells stored two to five different hormones. Multifunctional AP cells were much more abundant in females than in males, indicating that the hormonal changes associated with the sexual cycle may promote transdifferentiation. As the phenotypic analysis was performed here after stimulation with HRHs, the fraction of polyhormonal cells might have been underestimated. With this limitation, the polyhormonal cells detected here responded to the HRHs less than the monohormonal ones, suggesting that they might contribute less than expected a priori to paradoxical secretion. Overall, our results reveal a striking sexual dimorphism, the female pituitary being much more plastic than the male pituitary.
The anterior pituitary (AP) plays a central role in control of the endocrine system through secretion of the different AP hormones. Five main cell types are present, somatotropes, mammotropes, corticotropes, thyrotropes and gonadotropes, which secrete growth hormone (GH), prolactin (PRL), adrenocorticotrophic hormone (ACTH), thyroid-stimulating hormone (TSH) and gonadotropins (follicle-stimulating hormone (FSH) and luteinizing hormone (LH)), respectively. AP hormone secretion is regulated by the hypothalamic releasing hormones (HRHs), thyrotropin-releasing hormone (TRH), gonadotropin-releasing hormone (GnRH or LHRH), growth hormone releasing hormone (GHRH) and corticotropin-releasing hormone (CRH). Stimulation of HRH receptors induces an increase of the cytosolic free Ca2+ concentration ([Ca2+]c) and hormone release (Luini et al. 1985; Gershengorn, 1986; Shangold et al. 1988; Kato et al. 1992). It is generally accepted that each HRH specifically stimulates secretion of a single AP hormone, but several reports in the last decade have challenged this hypothesis. It has been reported that some pituitary cells may store and release more than one AP hormone and that stimulation with a given HRH may promote secretion of a non-corresponding hormone (paradoxical secretion). Mammosomatotropes that store and release GH and PRL (Frawley & Boockfor, 1991) have been regarded as an intermediate stage for conversion of somatotropes into mammotropes, a phenotypic switch between mature cell types without cell division termed transdifferentiation (Frawley & Boockfor, 1991). Polyhormonal corticotropes, co-storing ACTH and other AP hormones (Childs, 1991), somatogonadotropes co-storing GH and gonadotropins (Childs et al. 2000) and cells storing and releasing LH and PRL (Fukami et al. 1997) have also been reported. In addition, GH cells can transdifferentiate into thyrosomatotropes, containing both GH and TSH, after thyroidectomy (Horvath et al. 1990) or protracted primary hypothyroidism (Vidal et al. 2000).
On the other hand, a large population of rat AP cells bears multiple HRH receptors (multiresponsive cells, Kasahara et al. 1994; Villalobos et al. 1996, 1997) and stimulation with different HRHs evokes paradoxical PRL secretion (Villalobos et al. 1997). Gonadotropes may express GHRH receptors (Childs et al. 1999). Somatotropes may transiently express LHRH receptors and LHRH may stimulate GH secretion (Childs, 2000). In addition, single-cell RT-PCR has revealed that many pituitary cells from different species display mRNAs for multiple AP hormones (Roudbaraki et al. 1999; Seuntjens et al. 2002a, b; Okada et al. 2003; Hauspie et al. 2003). Overall, the above studies indicate that the AP contains multifunctional (multiresponsive and/or polyhormonal) cells whose stimulation may give rise to paradoxical secretion. Paradoxical secretory responses are common in non-normal pituitaries, especially pituitary tumours (Matsukura et al. 1977; De Marinis et al. 1990; Barlier et al. 1997), but they have also been sporadically reported in normal pituitaries, both in vitro and in vivo, including healthy humans (Amsterdam et al. 1982; Harvey, 1990).
Despite all the evidence indicating that the normal AP contains cells with mixed phenotypes involved in gland plasticity, a comprehensive characterization of the different AP cell phenotypes is lacking and the nature and physiological role of the multifunctional cells remains obscure. This is probably due to the difficulty of typing individual AP cells by the hormones they store (up to six) and the HRH receptors they express (up to four). Here we have attempted a complete phenotypic characterization of AP cells by assessing the expression of functional HRH receptors and the contents of AP hormones in the same individual cells. This was achieved by imaging Ca2+ responses to the four classic HRHs, followed by multiple sequential primary immunofluorescence (MSPI) typing of the six AP hormones (Alarcón & García-Sancho, 2000). Most previous work has been carried out in the rat, but the mouse was chosen here in order to provide the information needed for future studies in genetically modified animals, for which this species is more convenient.
METHODS
Antisera against mouse PRL (no. AFP131078Rb), rat β-TSH (no. AFP1274789), rat GH (no. AFP411S), rat β-FSH (no. AFPHSFSH6Rb), rat β-LH (no. AFP571292393R) and rat ACTH (no. AFP71111591GP) were generous gifts from the National Hormone and Pituitary Program (NHPP), the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Child Health and Human Development and the US Department of Agriculture through Dr A. F. Parlow. The anti-rat reagents work in the mouse just as well as in the rat (NHPP and A. F. Parlow, personal communication). Fluorescent antibodies were prepared by labelling with Oregon Green 488, Cascade Yellow or Alexa Fluor 350. Briefly, antibodies (10 mg ml−1) were incubated with the amine-reactive compounds at pH 9 (isothiocyanate) or 8.3 (succinimidyl esters) for 60 min. The reaction was terminated by adding excess hydroxylamine and the sample was desalted over a Sephadex G-25 column (for further details refer to Molecular Probes, amine-reactive probes product information sheet). The labelled antibodies were then purified over a protein A-Sepharose column (Harlow & Lane, 1988; Alarcón & García-Sancho, 2000). Fura-2 AM, Oregon Green 488-isothiocyanate, and the succinimidyl esters of Cascade Yellow and Alexa Fluor 350 were purchased from Molecular Probes Europe. The HRHs were obtained from Sigma.
Male and randomly cycling female mice (Balb/c, 12-weeks-old) were killed by cervical dislocation following the procedure approved by the Valladolid University animal ethics committee. After decapitation, the anterior pituitary glands were quickly removed and dispersed with 1 mg ml−1 trypsin (Sigma) in minimum essential medium (S-MEM; Gibco) for 30 min at 37 °C. Dispersed cells were plated onto coverslips previously coated with 0.01 mg ml−1 poly-l-lysine and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10 % fetal bovine serum and antibiotics. Experiments were performed after 2–6 h of culture. We have previously shown that responses to the HRHs by these cells are better than those by cells maintained in primary culture for 1–3 days (Villalobos et al. 1996, 1997).
Responsiveness of single cells to the four HRHs was assessed from their changes of [Ca2+]c, which were measured by digital imaging fluorescence microscopy as described previously (Villalobos et al. 1996, 1997). Briefly, cells were loaded with fura-2 AM (4 μm) for about 1 h at room temperature in standard medium of the following composition (mm): NaCl, 145; KCl, 5; MgCl2, 1; CaCl2, 1; Hepes, 10; pH 7.4; glucose, 10. Cells were then washed with the same medium, placed in a thermostatically controlled (37 °C) chamber on the stage of an inverted microscope (Nikon Diaphot) and perfused with standard medium, prewarmed at 37 °C. Cells were epi-illuminated alternately at 340 and 380 nm, and light emitted above 520 nm was recorded by using a Magical image processor (Applied Imaging, Newcastle, UK). Pixel by pixel ratios of consecutive frames were produced and [Ca2+]c was estimated from these ratios by comparison with fura-2 standards. Test solutions containing HRHs (10 nm) were perfused over 30 s at the times indicated. A depolarizing solution containing high K+ (75 mm; replacing an equi-osmotic amount of Na+) was perfused over 15 s at the end of the experiment in order to asses normal responsiveness of each cell preparation. Cells not responding to high K+ (about 5 % of the whole population) were excluded from the analysis.
The hormonal contents of the cells were typed by multiple sequential primary immunofluorescence (MSPI, Alarcón & García-Sancho, 2000). At the end of the [Ca2+]c measurements, cells were fixed with 4 % paraformaldehyde in phosphate buffered saline (PBS) for 10 min, permeabilized with 0.3 % Triton X-100 in the above solution for 3 min and washed with PBS. All washings were performed by perfusion with PBS at 2–3 ml min−1 for 5 min. Then 10 % goat serum in PBS was added to saturate non-specific binding sites. After 5 min, antibodies against three AP hormones (TSH, FSH and LH), labelled with Oregon Green 488, Cascade Yellow and Alexa Fluor 350, respectively, were added and the incubation was continued for 30 min. After washing, specific fluorescence images corresponding to each fluorophore were captured to reveal stained cells. The following fluorescence settings were used: Oregon Green (FSH): excitation 490 nm, emission > 510 nm; Cascade Yellow (TSH): excitation 380 nm, emission > 510 nm; Alexa Fluor 350 (LH): excitation 340 nm, emission 460 ± 20 nm. These three fluorophores were chosen because their fluorescence spectra allow complete separation of their signals at the above fluorescence settings. This step enables cells which store FSH, TSH or LH to be typed, as well as cells co-storing combinations of these AP hormones. After capturing the first series of images, cells were washed and incubated again with antibodies against GH, PRL and ACTH, labelled with Oregon Green 488 (PRL), Cascade Yellow (GH) or Alexa Fluor 350 (ACTH), and the incubation was continued for 30 min. After washing, three new fluorescence images were taken with the same fluorescence settings described above. This new series of images revealed cells stained by the second set of antibodies in addition to those stained with the first set. To reveal the specific staining by the second set of antibodies, the first series of fluorescence images were subtracted from the second ones. Figure 1 illustrates the procedure. Only one of the fluorescent channels is shown for simplicity. In preliminary tests, dilutions of the different antibodies were adjusted to yield similar fluorescence staining. In order to simplify image arithmetics, all the images were taken at the same camera gain and neutral density filters of known absorbance were used when required. This allowed us to avoid image saturation and to expand the useful dynamic range of measurements. The top and middle panels in Fig. 2A illustrate six images corresponding to each cell type after image processing. Control experiments were performed to show that: (i) fluorescence staining with the different antibodies did not decrease from the first to the second round of measurements, (ii) similar results were obtained when antibodies were conjugated with different fluorophores or used in different sequences, and (iii) omission and/or substitution of the antibody in each sequence did not modify the results. Once the images for the different antibodies were taken, nuclei were stained with Hoechst 33258 (0.5 μg ml−1, 10 min; Fig. 2A centre panel, bottom row) and another fluorescence image was acquired (excitation 340 nm; emission 450 nm). The image from the fluorescence-stained nuclei facilitated definition of cellular boundaries in cells that were physically close.
Figure 1. Image arithmetics in multiple sequential primary immunofluorescence.
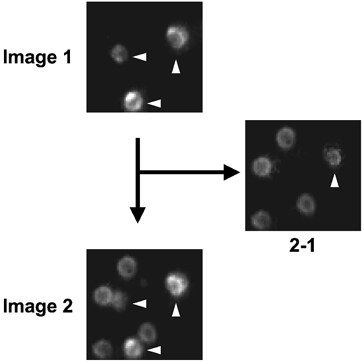
Image 1 was taken after incubation with the FSH–Cascade Yellow antibody, which stained three cells in the field. Image 2 was taken after the subsequent incubation with the PRL–Cascade Yellow antibody; the same three cells as in Image 1 plus five new cells are now visible. Only one of the FSH-positive cells, that signalled by the vertical arrowhead, had increased staining from Image 1 to Image 2. Subtraction of Image 1 from Image 2 (2 − 1) reveals the PRL-positive cells, which include one cell which also contains FSH (vertical arrowhead).
Figure 2. Combination of calcium imaging and multiple sequential primary immunofluorescence (MSPI).
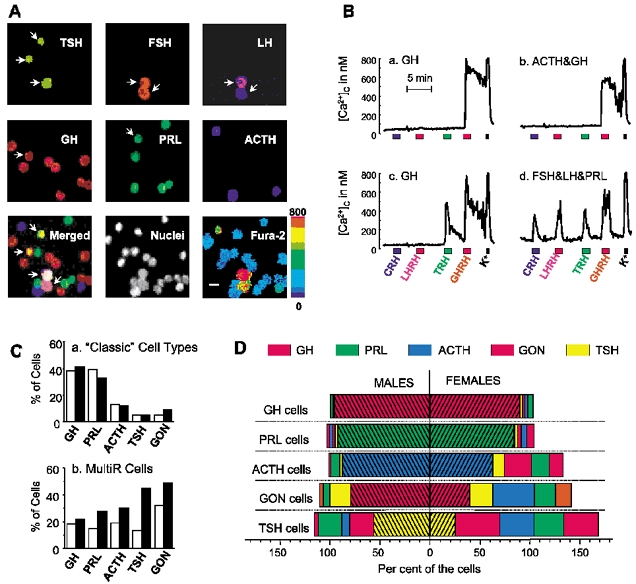
A, mouse AP cells were loaded with fura-2 and single-cell [Ca2+]c measurements were performed by digital imaging fluorescence microscopy. Cells were then fixed and subjected to MSPI (see Methods for details). The upper and middle rows show staining with the six antibodies against the different AP hormones. The grey images have been coloured with different colours. The bottom row shows, from left to right, (i) the merger image of the above six images; (ii) fluorescence of nuclei, stained with Hoechst 33258; and (iii) fura-2 ratio image taken during stimulation with LHRH (pseudocolour-coded for [Ca2+], from dark blue to red; 0–800 nm, scale on right). The arrows point to polyhormonal cells. Calibration bar, 10 μm. B, representative [Ca2+]c traces of individual AP cells in response to sequential addition of the four HRHs (10 nm) and high-K+ solution (75 mm). Cells were typed thereafter by MSPI and the resulting stored hormones are indicated above each trace. Traces correspond to different cells storing only GH (a and c), ACTH and GH (b) or FSH, LH and PRL (d). C, relative abundance of ‘classic’ cell types (a, as percentages of all the hormone-containing cells) and multiresponsive cells (b, as percentages of all the cells in each ‘classic’ cell type) in male- (open bars) and female- (filled bars) derived mouse AP cells. GON, gonadotropes storing both FSH and LH. Data from 701 male cells and 558 female cells from 11 different experiments. D, hormonal contents within each one of the different ‘classic’ AP cell types in male (left) and female mice (right). The hatched bars represent the population of monohormonal cells. All the other bars correspond to polyhormonal cells containing both the type-defining and the indicated co-stored hormones. The addition of all the percentages may be larger than 100 % because some cells actually contained more than two hormones.
RESULTS
Figure 2A summarizes the novel strategy used for characterization of the cell phenotypes present in primary cultures of mouse AP cells. First, fura-2-loaded cells were subjected to Ca2+ imaging during sequential stimulation with the four HRHs (bottom right image). Then, cells in the same microscopic field were fixed and subjected to MSPI (top and middle rows in Fig. 2A; see Methods for details). The merger of the six MSPI images is shown in the leftmost image of the bottom row. Arrows identify four polyhormonal cells present in the field. Total cells were counted by nucleus staining (centre image of the bottom row). This procedure enables typing of every individual cell by responses to four HRHs and storage of the six AP hormones. Figure 2B shows four representative examples of cell phenotypes ([Ca2+]c responses and hormone storage). We found cells that responded to only one HRH and stored one single AP hormone (Fig. 2Ba). The cell shown here can be considered as an orthodox somatotrope. We found also orthodox examples of mammotropes (storing PRL and responding only to TRH), corticotropes (storing ACTH and responding only to CRH), thyrotropes (storing TSH and responding only to TRH) and gonadotropes (storing LH and FSH and responding only to LHRH). In addition, we found cells responsive to a single HRH that stored more than one AP hormone (Fig. 2Bb) as well as cells that were responsive to several HRHs (multiresponsive cells), either storing one single AP hormone (Fig. 2Bc) or more than one (Fig. 2Bd). The cell phenotypes falling into the last three categories cannot be considered orthodox AP cells and will be referred to as ‘non-classic’. We also found cells storing one single AP hormone and expressing a non-related HRH receptor (for example, a cell expressing ACTH and responding only to LHRH), which can likewise be considered non-classic. Thus, mouse AP cells may express multiple combinations of AP hormones and HRH receptors.
Table 1 summarizes the results of 701 male cells and 558 female cells, classified according to the number of AP hormones stored (single hormone, more than one hormone (polyhormonal) or no hormone) and the number of HRH receptors expressed (single receptor, more than one receptor (multiresponsive) or no receptors). The multiresponsive cells (19–38 %) were more abundant than the polyhormonal cells (8–13 %), suggesting that both cell populations do not necessarily overlap. In addition, the percentages of both multiresponsive and polyhormonal cells were about twice as large in females as in males.
Table 1.
Proportional abundance (percentage of all cells) of different cell phenotypes in the male and female mouse anterior pituitary
| HRH receptor | ||||||||
|---|---|---|---|---|---|---|---|---|
| Monoreceptor | Multireceptor | No receptor | Σ | |||||
| M | F | M | F | M | F | M | F | |
| AP hormone | ||||||||
| Monohormonal | 43 | 34 | 16 | 31 | 23 | 16 | 84 | 81 |
| Polyhormonal | 3 | 4 | 2 | 5 | 3 | 4 | 8 | 13 |
| No hormone | 1 | 3 | 1 | 2 | 6 | 1 | 8 | 6 |
| Σ | 47 | 41 | 19 | 38 | 34 | 21 | 100 | 100 |
Cells were classified according to the number of HRH receptors and AP hormones. Data derived from 11 independent experiments (701 and 558 single mouse AP cells from males and females, respectively). M, male; F, female.
Figure 2Ca shows the size of the ‘classic’ AP cell subpopulations, pooled according to the hormone they stored, regardless of the number of stored hormones. Cells storing either GH or PRL made up 80–88 % of the whole population. The remaining 12–20 % stored ACTH, TSH, LH or FSH. Most of the gonadotropes (65–85 %) co-stored both gonadotropins, FSH and LH, and will be considered here as a single cell type (GON cells). Therefore, when AP cells were typed in this classic manner, the frequency pattern was similar for male and female cells, and similar to the pattern reported previously for rats (Villalobos et al. 1996). It could be argued that, since hormone typing was performed here after stimulation with the HRHs and high K+, some cells could degranulate, thus decreasing the value of the estimated frequency. Preliminary experiments performed in the same batches of cells with and without stimulation with the HRHs showed no significant differences in the frequencies of the different AP cell types (results not shown), but we cannot exclude the possibility that this procedure may actually underestimate the proportion of polyhormonal cells. Figure 2Cb shows the proportion of multiresponsive cells within each classic cell type. They amounted to 13–30 % of each cell type in males and 22–50 % in females. Thus, many AP cells exhibit mixed cell phenotypes and the relative abundance of both polyhormonal and multiresponsive cells depended largely on the cell type and on the gender of the pituitary donor.
Some cells actually stored more than one AP hormone, but the percentage of polyhormonal cells was largely dependent on the cell type. Figure 2D summarizes the results of the analysis for hormone storage in males (left bars) and females (right bars). Within each classic cell type, the percentage of monohormonal cells is represented by the hatched bars adjacent to the ordinate axis. The other bars represent the percentage of cells containing, in addition, other co-stored hormones. The sum of all the cell fractions is frequently more than 100 %. This is because some cells actually contained more than two hormones, and therefore they were included in more than one cell fraction. It is clear that the profiles of hormonal content varied among different cell types and also between male- and female-derived cells.
In somatotropes and mammotropes (GH cells and PRL cells, top two pairs of bars in Fig. 2D) the hormonal profiles were rather orthodox, and very similar in males and females. Most somatotropes (91–95 %) and mammotropes (85–92 %) were monohormonal. In polyhormonal cells, the co-stored hormone was distributed quite randomly among all the other AP hormones.
The hormonal contents of corticotropes were very different in male and female mice cells. Most of the male corticotropes were monohormonal (87 %) and polyhormonal corticotropes co-stored either PRL (9 % of the cells), GH (2 %) or TSH (2 %). No corticogonadotropes were found. In female corticotropes, almost 40 % were polyhormonal and the most frequent cell type in this category was the corticogonadotrope (27 % of the cells), although the percentage of cells containing other AP hormones, PRL, GH and TSH, was significant (12–19 % of the cells).
The differences between male and female cells were even more striking for gonadotropes. Most male gonadotropes stored only gonadotropins (79 % of all the cells). The polyhormonal gonadotropes co-stored either TSH (21 %), PRL (7 %) or GH (4 %) together with FSH and LH. In the female, gonadotropes storing just FSH and LH were only 40 %. A larger fraction (42 %) co-stored ACTH (note that this cell type was not found in the male) and 16–24 % co-stored either TSH, PRL or GH.
The most surprising outcome was revealed by the analysis of thyrotropes. Even in the male, the polyhormonal cells were almost 50 % of the total cells. They co-stored most frequently gonadotropins and PRL (24 % of the cells each one). A smaller percentage (4–8 %) co-stored ACTH or GH. In the female, only 25 % of the thyrotropes were monohormonal. The percentage of polyhormonal cells co-storing the other four hormones, gonadotropins, ACTH, GH and PRL, each amounted to 30–45 % of the cells.
Some AP cells stored three or more hormones. In the male, trihormonal cells were rare (< 2 %) in all the cell types except thyrotropes, where they amounted to 31 % of this category. In females, trihormonal cells were rare among somatotropes and thyrotropes (< 6 %), but very common in the other cell types (30–45 %). In females, 10 % of the thyrotropes stored as many as five different hormones.
In order to assess the functional consequences of polyhormonality it is pertinent to ask whether the co-stored hormones are present in just trace amounts or in amounts comparable to those of monohormonal cells. To estimate the amount of stored hormones, the fluorescence of the staining antibody was quantified for every single cell and standardized in each experiment by referring its value as a fraction of the average value found for the corresponding monohormonal cell kind. The contents of the kind-defining hormone in monohormonal and polyhormonal cells are compared in Fig. 3. Data from males and females have been pooled in this figure, as there were no major differences between genders. Surprisingly, the hormone content for each hormone was, in all the cases, very similar for the monohormonal and the polyhormonal cells. In addition, the s.e.m. values were relatively small indicating that the population was rather homogeneous. Previous work demostrated that co-storage of two hormones usually correlated with co-release (Frawley & Boockfor, 1991; Fukami et al. 1997). Thus, our results suggest that considerable mix-up of hormones could take place when polyhormonal cells are stimulated to secrete.
Figure 3. Comparison of the hormonal contents of monohormonal and polyhormonal cells.
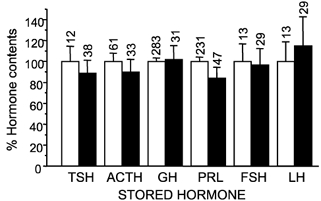
Contents of the type-defining hormone are expressed as a percentage of the contents of the monohormonal cell pool of the same experiment. Means ± s.e.m. values are shown. The number of individual cells analysed in each case is shown above the bars. FSH and LH refer to the amounts of each one of these hormones in gonadotropes containing both hormones either alone (monohormonal, □) or in addition to another co-stored hormone (polyhormonal, ▪).
Hypothalamic releasing hormones are the major factors for control of AP secretion. The actions of HRHs at the single-cell level were quantified here from their effects on [Ca2+]c. In order to compare the magnitudes of the Ca2+ responses to the HRHs in the different cell populations, the maximum [Ca2+]c increase observed during the 60 s period following stimulation with HRH (Δ[Ca2+]c) was measured in each single cell. Then the cells were grouped by the hormones they contain (TSH, GON, ACTH, PRL or GH, determined by MSPI at the end of the [Ca2+]c measurements), subdivided into monohormonal or polyhormonal, and the mean Δ[Ca2+]c and the s.e.m. values were calculated for each group. The output was similar for males and females, so that data from both sexes were pooled (Fig. 4). It is clear that the strongest responses to each HRH were observed in the monohormonal group of the corresponding cell kind: corticotropes for CRH, gonadotropes for LHRH, thyrotropes for TRH and somatotropes for GH. The responses in all the other groups, including polyhormonal cells of the corresponding cell kind, were much smaller. Mammotropes showed little specificity regarding their responses to HRHs. In this, mouse mammotropes differ from their rat counterparts, which are especially responsive to TRH (Villalobos et al. 1996, 1997). Cells containing no hormones were rarely sensitive to HRHs (results not shown).
Figure 4. Calcium responses of the different monohormonal and polyhormonal cell types to the hypothalamic releasing factors.
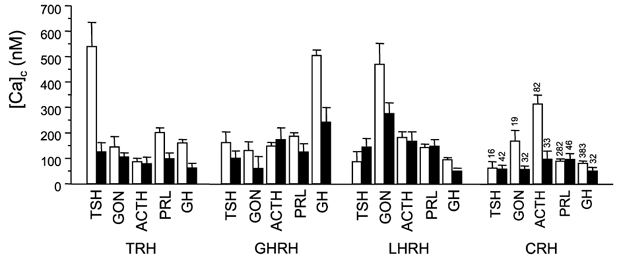
Responses are expressed as Δ[Ca2+] (maximum increase in [Ca2+]c during the stimulation period) to the HRH in each cell type. Bars represent means ± s.e.m.; □, monohormonal; ▪, polyhormonal. The figures above the bars on the right indicate the number of cells measured. Data from 701 male and 558 female cells are from 11 different experiments.
Responses to HRHs were also analysed in terms of the percentage of cells responding to each HRH within every cell population. The results were generally consistent with those for Δ[Ca2+]c, but polyhormonal cells seemed somewhat more responsive when analysed in this way, especially in females. In particular, the percentage of responding cells was similar in monohormonal and polyhormonal gonadotropes, and responses to LHRH were also found in many polyhormonal thyrotropes and corticotropes, but polyhormonal cells tended to show smaller Δ[Ca2+]c responses than their monohormonal counterparts.
Monohormonal cells responding to HRHs other than the corresponding ones should also be regarded as ‘non-classic’. Among somatotropes only 5 % of the cells responded to HRHs other than GH. In thyrotropes, corticotropes and gonadotropes the percentage of monohormonal cells responding to non-corresponding HRHs was 30–32 %. For mammotropes the profile of sensitivity to HRHs was rather non-specific, with GHRH and TRH being somewhat more effective than the other HRHs (results not shown).
DISCUSSION
The existence of polyhormonal and/or multiresponsive cells may have important implications for the physiology of the AP, particularly in relation to paradoxical secretion and transdifferentiation. Here we present, for the first time, a complete phenotypic characterization of the AP cell populations in male and cycling female mice according to the six AP hormones stored and the four HRH receptors expressed by individual cells. This landscape view allows us to propose some general principles.
In the two major cell subpopulations, somatotropes and mammotropes, polyhormonal cells were rare, but they were more frequent in corticotropes and gonadotropes, and in thyrotropes they were the rule rather than the exception. Even though the relative abundance of the classic cell type was similar for male and female mice, multifunctional cell phenotypes were much more frequent in female pituitaries (Fig. 2C and D). With the usual reservations for in vitro experiments, this outcome suggests that plasticity of the pituitary is much greater in females than males. Further research is nevertheless required to support the role of sex differences in transdifferentiation in the in vivo situation, for example by studying the effects of castration and sex hormone replacement on polyhormonality.
Polyhormonal cells containing several combinations of two different AP hormones have been reported previously (see Introduction). The study presented here shows that all the 10 possible two-hormone combinations of the five AP hormones are present in the mouse pituitary. Some cells stored three or more hormones. The amounts of hormones co-stored in polyhormonal cells were similar to those found in the corresponding monohormonal cells, suggesting that they could significantly contaminate the secretory output and contribute to paradoxical secretion. However, speculations on the contribution of polyhormonal cells to paradoxical secretion should take into account the relative frequencies of the different hormone combinations. Thus, paradoxical contribution to GH and PRL secretion is unlikely, as polyhormonal cells are a very small percentage of somatotropes and mammotropes. Taking this view, paradoxical secretion of TSH or gonadotropines should be more likely, as these cell types contain the largest fraction of polyhormonal cells.
The distribution of HRH receptors should be, perhaps, even more relevant for paradoxical secretion. We have reported earlier that about 30 % of all male rat AP cells bear multiple HRH receptors (Villalobos et al. 1997). Here we find that about 19 % of all male cells and as much as 38 % of female cells express multiple HRH receptors in the mouse. The relative abundance of multiresponsive cells was also influenced by the cell lineage, being larger in those cell populations showing less proportional abundance (up to nearly 50 % in female thyrotropes and gonadotropes). Most of the monohormonal cells of each type responded mainly to their corresponding HRH. In contrast, the specificity was lost in polyhormonal cells, which responded poorly to all the four HRHs (Fig. 4). Although we cannot equate [Ca2+] responses and secretion, these results suggest that contribution of polyhormonal cells to the secretory responses should be less important than that of monohormonal cells. This may explain why paradoxical secretion has been so elusive. Nevertheless, it should be remarked that the phenotypic analysis performed here was carried out after sequential stimulation with four secretagogues and high K+. This could result in an underestimation of the proportional abundance of multihormonal cells as we cannot be positively certain that monohormonal cells were actually monohormonal before stimulation. In addition, the situation could be different in particular physiological or pathophysiological situations where a specific increase of a given fraction of multifunctional cells takes place. For example, GH secretion evoked by LHRH from somatogonadotropes may be negligible in males or randomly cycling females, but become relevant during pro-oestrous (Childs et al. 2000); or PRL secretion from mammosomatotropes may become significant during lactation (Frawley & Boockfor, 1991). Thus, paradoxical secretion from polyhormonal and/or multiresponsive cells could be quantitatively significant in particular physiological and pathophysiological conditions, such as some phases of the oestrous cycle, the beginning of lactation, protracted hypothyroidism, etc.
Cells expressing mRNAs for multiple AP hormones have been reported in the rat (Childs et al. 2000; Seuntjens et al. 2002a, b) and mouse (Seuntjens et al. 2002a, b). Even though high percentages of these cells can be detected (16–36 %), most of them do not translate these mRNAs to the respective hormones (Roudbaraki et al. 1999). Cells containing multiple hormone mRNAs have been proposed to function as ‘reserve cells’ that can quickly generate a particular polyhormonal cell containing a new hormone by activation of translation when physiologically required (Seuntjens et al. 2002a, b). Alternatively, cells with mixed phenotypes may arise by transdifferentiation from mature cells. Frawley & Boockfor (1991) proposed that the mammosomatotrope cell lineage was an intermediate step in the conversion of somatotropes into mammotropes. More recently, Childs and coworkers proposed that somatotropes are able to develop into somatogonadotropes and acquire the LHRH receptor during the oestrous cycle in a process involving stimulation of the oestrogen receptor (Childs, 2002). Oestrogens may also promote transdifferentiation from somatotropes to mammotropes (Porter et al. 1992; Horvath & Schally, 1994). Our results suggest that transdifferentiation during the sexual cycle may also occur in all other cell types, as the increase of mixed cell phenotypes in females is a general occurrence (Fig. 2D). However, the large proportion of polyhormonal and multiresponsive thyrotropes found in males suggest that additional factors, independent of sexual cycle, may be involved in promoting transdifferentiation. According to our results, polyhormonal cells are generally poorly responsive to HRHs. Under this view, completion of transdifferentiation towards a new monohormonal phenotype should involve a further step with loss of some of the stored hormones and acquisition of a response to a new HRH. This step may be controlled by different regulatory factors. The relative abundance of the different regulatory factors would therefore determine the actual composition of multifunctional cells at any one time.
All the mixed cell phenotypes were present in comparable proportional abundance (between 0.6 and 1.8 %) when expressed as percentages of the total number of cells. If mixed phenotypes were generated by transdifferentiation from mature cells, then thyrotropes, gonadotropes and corticotropes would tend to transdifferentiate more often than somatotropes and mammotropes. On the other hand, if we consider that transdifferentiation is directed by different external signals for each cell type, then the simultaneous influence of two signals should be invoked in order to promote transdifferentiation into polyhormonal cells storing three or more hormones. In an alternative model, mixed cells could arise from a specific kind of pluripotential cell storing multiple hormones. At present, we have no evidence to favour either of these two alternative mechanisms for generation of multifunctional cells. Whichever the situation, our results indicate that the fraction of cells with mixed phenotypes can change under physiological conditions.
Acknowledgments
We thank the National Hormone and Peptide Programme and Dr A. F. Parlow for providing antisera against AP hormones, and J. Fernández for technical help. This work was supported by grants from the Spanish Ministerio de Ciencia y Tecnología (BFI 2001–2073 to J.G.S.) and the Fondo de Investigaciones Sanitarias (01/0769 to C.V.). L.N. and C.V. are fellows of the Ramon y Cajal Program of the Spanish Ministerio de Ciencia y Tecnología. L.S. holds a predoctoral fellowship from the Spanish Ministerio de Ciencia y Tecnología.
L. Núñez and C. Villalobos contributed equally to this work
References
- Alarcón P, García-Sancho J. Differential calcium responses to the pituitary adenylate cyclase-activating polypeptide (PACAP) in the five main cell types of rat anterior pituitary. Pflugers Arch. 2000;440:685–691. doi: 10.1007/s004240000368. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Winokur A, Lucki I, Snyder P, Harris RI, Caroff S, Rickels K. Growth hormone, prolactin and thyrotropin responses to gonadotropin-releasing hormone in depressed patients and healthy volunteers. Psychoneuroendocrinology. 1982;7:177–184. doi: 10.1016/0306-4530(82)90010-5. [DOI] [PubMed] [Google Scholar]
- Barlier A, Pellegrini-Bouiller I, Caccavelli L, Gunz G, Morange-Ramos I, Jaquet P, Enjalbert A. Abnormal transduction mechanisms in pituitary adenomas. Horm Res. 1997;47:227–234. doi: 10.1159/000185468. [DOI] [PubMed] [Google Scholar]
- Childs GV. Multipotential pituitary cells that contain ACTH and other pituitary hormones. Trends Endocrinol Metab. 1991;2:112–117. doi: 10.1016/s1043-2760(05)80007-4. [DOI] [PubMed] [Google Scholar]
- Childs GV. Growth hormone cells as co-gonadotropes: partners in the regulation of the reproductive system. Trends Endocrinol Metab. 2000;11:168–175. doi: 10.1016/s1043-2760(00)00252-6. [DOI] [PubMed] [Google Scholar]
- Childs GV. Development of gonadotropes may involve cyclic transdifferentiation of growth hormone cells. Arch Physiol Biochem. 2002;110:42–49. doi: 10.1076/apab.110.1.42.906. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Miller BT, Collins J. Differential expression of gonadotropin and prolactin antigens by GHRH target cells from male and female rats. J Endocrinol. 1999;162:177–187. doi: 10.1677/joe.0.1620177. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Wu P. Differential expression of growth hormone messenger ribonucleic acid by somatotropes and gonadotropes in male and cycling female rats. Endocrinology. 2000;141:1560–1570. doi: 10.1210/endo.141.4.7429. [DOI] [PubMed] [Google Scholar]
- De Marinis L, Mancini A, Zuppi P, Anile C, Maira G. Paradoxical growth hormone response to thyrotropin-releasing hormone in acromegaly. Clinical correlations and prognostic value. Acta Endocrinol (Copenh) 1990;122:443–449. doi: 10.1530/acta.0.1220443. [DOI] [PubMed] [Google Scholar]
- Frawley LS, Boockfor FR. Mammosomatotropes: presence and functions in normal and neoplastic pituitary tissue. Endocr Rev. 1991;12:337–355. doi: 10.1210/edrv-12-4-337. [DOI] [PubMed] [Google Scholar]
- Fukami K, Tasaka K, Mizuki J, Kasahara K, Masumoto N, Miyake A, Murata Y. Bihormonal cells secreting both prolactin and gonadotropins in normal rat pituitary cells. Endocr J. 1997;44:819–826. doi: 10.1507/endocrj.44.819. [DOI] [PubMed] [Google Scholar]
- Gershengorn MC. Mechanism of thyrotropin releasing hormone stimulation of pituitary hormone secretion. Annu Rev Physiol. 1986;48:515–526. doi: 10.1146/annurev.ph.48.030186.002503. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. In Antibodies: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 1988. p. 310. [Google Scholar]
- Harvey S. Thyrotropin-releasing hormone: a growth hormone-releasing factor. J Endocrinol. 1990;125:345–358. doi: 10.1677/joe.0.1250345. [DOI] [PubMed] [Google Scholar]
- Hauspie A, Seuntjens E, Vankelecom H, Denef C. Stimulation of combinatorial expression of prolactin and glycoprotein hormone α-subunit genes by gonadotropin-releasing hormone and estradiol-17β in single rat pituitary cells during aggregate cell culture. Endocrinology. 2003;144:388–399. doi: 10.1210/en.2002-220606. [DOI] [PubMed] [Google Scholar]
- Horvath E, Lloyd RV, Kovacs K. Propylthiouracyl-induced hypothyroidism results in reversible transdifferentiation of somatotrophs into thyroidectomy cells. A morphologic study of the rat pituitary including immunoelectron microscopy. Lab Invest. 1990;63:511–520. [PubMed] [Google Scholar]
- Horvath JE, Schally AV. Reciprocal changes in prolactin and growth hormone secretion in vitro after in vivo estrogen treatment. Acta Biol Hung. 1994;45:249–262. [PubMed] [Google Scholar]
- Kasahara K, Tasaka K, Masumoto N, Mizuki J, Tahara M, Miyake A, Tanizawa O. Characterization of rat pituitary cells by their responses to hypothalamic releasing hormones. Biochem Biophys Res Commun. 1994;1999:1436–1441. doi: 10.1006/bbrc.1994.1391. [DOI] [PubMed] [Google Scholar]
- Kato M, Hoyland J, Sikdar SK, Mason WT. Imaging of intracellular calcium in rat anterior pituitary cells in response to growth hormone releasing factor. J Physiol. 1992;447:171–189. doi: 10.1113/jphysiol.1992.sp018997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A, Lewis D, Guild S, Corda D, Axelrod J. Hormone secretagogues increase cytosolic calcium by increasing cAMP in corticotropin-secreting cells. Proc Natl Acad Sci U S A. 1985;82:8034–8038. doi: 10.1073/pnas.82.23.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura S, Kakita T, Hirata Y, Yoshimi H, Fukase M. Adenylate cyclase of GH and ACTH producing tumors of human: activation by non-specific hormones and other bioactive substances. J Clin Endocrinol Metab. 1977;44:392–397. doi: 10.1210/jcem-44-2-392. [DOI] [PubMed] [Google Scholar]
- Okada Y, Fujii Y, Moore JP, Jr, Winters SJ. Androgen receptors in gonadotrophs in pituitary cultures from adult male monkeys and rats. Endocrinology. 2003;144:267–274. doi: 10.1210/en.2002-220770. [DOI] [PubMed] [Google Scholar]
- Porter TE, Ellerkmann E, Frawley LS. Acute recruitment of prolactin-secreted cells is regulated posttranscriptionally. Mol Cell Endocrinol. 1992;84:23–31. doi: 10.1016/0303-7207(92)90067-g. [DOI] [PubMed] [Google Scholar]
- Roudbaraki M, Lorsignol A, Langouche L, Callewaert G, Vankelecom H, Denef C. Target cells of γ3-melanocyte-stimulating hormone detected through intracellular Ca2+ responses in immature rat pituitary constitute a fraction of all main pituitary cell types, but mostly express multiple hormone phenotypes at the messenger ribonucleic acid level. Refractoriness to melanocortin-3 receptor blockade in the lacto-somatotroph lineage. Endocrinology. 1999;140:4874–4885. doi: 10.1210/endo.140.10.7080. [DOI] [PubMed] [Google Scholar]
- Seuntjens E, Hauspie A, Roudbaraki M, Vankelecom H, Denef C. Combined expression of different hormone genes in single cells of normal rat and mouse pituitary. Arch Physiol Biochem. 2002a;110:12–15. doi: 10.1076/apab.110.1.12.904. [DOI] [PubMed] [Google Scholar]
- Seuntjens E, Hauspie A, Vankelecom H, Denef C. Ontogeny of plurihormonal cells in the anterior pituitary of the mouse, as studied by means of hormone mRNA detection in single cells. J Neuroendocrinol. 2002b;14:611–619. doi: 10.1046/j.1365-2826.2002.00808.x. [DOI] [PubMed] [Google Scholar]
- Shangold GA, Murphy SN, Miller RJ. Gonadotropin-releasing hormone-induced Ca2+ transients in single identified gonadotropes require both intracellular Ca2+ mobilization and Ca2+ influx. Proc Natl Acad Sci U S A. 1988;85:6566–6570. doi: 10.1073/pnas.85.17.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S, Horvath E, Kovacs K, Cohen SM, Lloyd RV, Scheithauer BW. Transdifferentiation of somatotrophs to thyrotrophs in the pituitary of patients with protracted primary hypothyroidism. Virchows Arch. 2000;436:43–51. doi: 10.1007/pl00008197. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Núñez L, Frawley LS, García-Sancho J, Sánchez A. Multi-responsiveness of single anterior pituitary cells to hypothalamic-releasing hormones: A cellular basis for paradoxical secretion. Proc Natl Acad Sci U S A. 1997;94:14132–14137. doi: 10.1073/pnas.94.25.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Núñez L, García-Sancho J. Functional glutamate receptors in a subpopulation of anterior pituitary cells. FASEB J. 1996;10:654–660. doi: 10.1096/fasebj.10.5.8621065. [DOI] [PubMed] [Google Scholar]


