Abstract
Whole-cell recordings showed that, in mouse mammary C127 cells transfected with the full genome of the bovine papilloma virus (BPV), a hypotonic challenge induced the activation of outwardly rectifying Cl− currents with a peak amplitude 2.7 times greater than that in control C127 cells. Cell-attached single-channel recordings showed that BPV-induced augmentation of the peak amplitude of the whole-cell current could not chiefly be explained by a small increase (1.2 times) in unitary conductance. There was no difference between control and BPV-transfected cells in the osmotic cell swelling rate, and hence, osmotic water permeability. However, a plot of the whole-cell current density as a function of cell volume, which was measured simultaneously, showed that the BPV-transfected cells had a strikingly greater volume sensitivity than control cells. Since the E5 protein of BPV has been reported to induce constitutive activation of the epidermal growth factor (EGF) receptor and platelet-derived growth factor (PDGF) receptor in a variety of cell lines including C127 cells, effects of the growth factors on volume-sensitive outwardly rectifying (VSOR) Cl− currents were examined in C127 cells. Application of PDGF peptides failed to affect the Cl− currents in control and BPV-transfected cells, although C127 cells are known to endogenously express PDGF receptors. In contrast, EGF peptides significantly increased the VSOR Cl− current in control cells. However, they failed to induce further augmentation of the current in BPV-transfected cells. VSOR Cl− currents were inhibited by tyrphostin B46, an inhibitor of the EGF receptor tyrosine kinase, in both control and BPV-transfected cells. The IC50 value in BPV-transfected cells (12 μm) was lower than that in control cells (31 μm). However, the VSOR Cl− currents in both cell types were insensitive to tyrphostin AG1296, an inhibitor of the PDGF receptor tyrosine kinase. The rate of regulatory volume decrease (RVD) was markedly diminished by tyrphostin B46 but not significantly affected by tyrphostin AG1296. We thus conclude that the EGF receptor tyrosine kinase upregulates the activity of the VSOR Cl− channel, mainly by enhancing the volume sensitivity.
Cells constantly go through osmotic transitions during their lifetime, since both intracellular metabolism and membrane transport produce fluctuations in the concentrations of osmotically active constituents. Cellular swelling in response to a hypo-osmotic challenge activates anion channels in most cell types (Strange et al. 1996; Nilius et al. 1997a; Okada, 1997). Volume-sensitive outwardly rectifying (VSOR) Cl− channels are expressed in a variety of cell types. Cell volume regulation in response to a hypotonic challenge has been recognised as a primary function of this type of channel (Strange et al. 1996; Nilius et al. 1997a; Okada, 1997). However, a number of recent studies have shown that VSOR Cl− channels are involved in other physiological processes, including intracellular acid–base balance due to permeability to lactate and bicarbonate ions (Nilius et al. 1998), the cell cycle (Shen et al. 2000) and apoptosis (Maeno et al. 2000; Okada et al. 2001). Also, swelling-activated Cl− channels play an important role in mechanisms controlling the proliferation of a variety of cultured cells (Voets et al. 1995; Nilius et al. 1997b; Rouzaire-Dubois & Dubois, 1998; Wondergem et al. 2001).
The precise activation mechanism of VSOR Cl− channels is as yet unknown. However, it is evident that VSOR Cl− channel activity involves one or more tyrosine phosphorylation steps, in the light of the following observations: (1) cell swelling induces activation of some protein tyrosine kinases (PTKs) in a number of cell types (Sadoshima et al. 1996; Tilly et al. 1996; Sinning et al. 1997; Crepel et al. 1998; Lepple-Wienhues et al. 1998; MacKenna et al. 1998), (2) a number of PTK antagonists inhibited swelling-induced 125I− efflux in Intestine 407 cells (Tilly et al. 1993), and swelling-activated Cl− conductance in other cell types (Sorota, 1995; Crepel et al. 1998; Lepple-Wienhues et al. 1998; Voets et al. 1998; Bryan-Sisneros et al. 2000; Shuba et al. 2000; Shen et al. 2001), (3) blockers of protein tyrosine phosphatase potentiated swelling-induced activation of Cl− currents in some cells (Voets et al. 1998; Shuba et al. 2000; Shen et al. 2001) and 125I− efflux in Intestine 407 cells (Tilly et al. 1993, 1994), and (4) introduction of purified PTK p56lck induced activation of outwardly rectifying Cl− currents in lymphocytes (Lepple-Wienhues et al. 1998).
Since receptor tyrosine kinases, especially growth factor receptors, are known to play a pivotal role in cell proliferation (Hubbard & Till, 2000), the possibility exists that VSOR Cl− channel activity is under the direct or indirect control of some growth factor receptor tyrosine kinase. In fact, an involvement of epidermal growth factor (EGF) receptor tyrosine kinase in the regulation of swelling-activated Cl− permeability (not Cl− conductance directly) was shown by monitoring swelling-induced 125I− efflux from Intestine 407 cells (Tilly et al. 1993). In the present study, this possibility was directly examined by comparing the VSOR Cl− currents in control mouse mammary C127 cells with those in C127 cells transfected with the full genome of the bovine papilloma virus (BPV), which has been demonstrated to induce cell growth transformation by constitutively activating tyrosine kinase-coupled receptors to EGF and platelet-derived growth factor (PDGF) (Martin et al. 1989; Petti et al. 1991; Cohen et al. 1993). Here, activation of EGF receptor tyrosine kinase was found to upregulate the VSOR Cl− channel activity, and the underlying mechanism was investigated.
A preliminary account of part of these results has appeared in abstract form (Abdullaev et al. 2001).
METHODS
Cells
A murine mammary cell line, C127, was obtained from the American Type Culture Collection (ATCC) and also kindly provided by Dr H. Cheng (Genzyme Corporation, Framingham, MA, USA). C127 cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10 % fetal calf serum (FCS). The amplitude and voltage dependence of swelling-activated whole-cell Cl− currents were indistinguishable between C127 cells obtained from ATCC (data shown below) and Genzyme (data not shown, n = 7). C127 cells, which had originally been obtained from ATCC, stably transfected with the pBPV vector (containing the full genome of the bovine papilloma virus) alone (BPV cells) or together with the ΔF508 mutant of the cystic fibrosis transmembrane conductance regulator (CFTR) (BPV/ΔF508 cells), were provided by Dr H. Cheng (Genzyme). C127 cells (which also originated from ATCC) stably transfected with wild-type (WT) CFTR and BPV (BPV/CFTR cells) were kindly provided by Dr M. J. Welsh (University of Iowa, IA, USA). Three types of BPV-transfected cells were cultured in DMEM with 10 % FCS and 200 μg ml−1 geneticin. In contrast to parental control cells, the three types of BPV-transfected cells exhibited more prominent proliferation, and did not exhibit contact inhibition.
Cells cultured on plastic flasks were resuspended by mechanical detachment, as reported previously (Kubo & Okada, 1992), and cultured with agitation for 15–300 min. Cells were then placed in a chamber and after they had attached to the glass bottom of the chamber were washed with bath solution. A hypotonic challenge was applied by switching from an isotonic to a hypotonic bath solution.
Electrophysiology
Whole-cell recordings were performed as reported previously (Kubo & Okada, 1992; Liu et al. 1998). Patch electrodes were fabricated from borosilicate glass capillaries using a micropipette puller (Sutter Instruments, Novato, CA, USA). Electrodes, filled with pipette solution, had a resistance of about 2 MΩ. Series resistance (< 5 MΩ) was compensated (70–80 %) to minimise voltage errors. Currents were recorded using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA, USA), filtered at 1 kHz using a four-pore Bessel filter and digitised at 4 kHz. pClamp software (version 6.0.2, Axon Instruments) was used for command pulse control, data acquisition and analysis. The time course of current activation and recovery was monitored by repetitively (every 15 s) applying alternating step pulses from a holding potential of 0 to ±40 mV. To observe the voltage dependence of current inactivation kinetics at large positive potentials, step pulses (2 s duration) were applied from a pre-potential of −100 mV to test potentials of −100 to +100 mV in 20 mV increments after reaching a steady-state level of swelling-activated current. The amplitude of instantaneous current was measured 1.25 ms after the step pulse onset. The current density was calculated by dividing the current amplitude by the cell capacitance (19.8 ± 0.9, 16.5 ± 2.0, 14.7 ± 0.8 and 15.3 ± 1.2 pF in control, BPV, BPV/CFTR and BPV/ΔF508 cells, respectively) which was little changed upon osmotic cell swelling, as reported in other cell types (Okada, 1997). The isotonic bath solution contained (mm): CsCl 110, CaCl2 2, MgCl2 1, Hepes 5, glucose 5 and mannitol 50. The pH was adjusted to 7.4 with CsOH. The osmolality of this solution was 275 ± 3 mosmol (kg H2O)−1. The hypotonic bath solution had the same composition as the isotonic solution but lacked mannitol, and its osmolality was 230 ± 3 mosmol (kg H2O)−1. The pipette solution contained (mm): CsCl 110, MgSO4 2, Na-Hepes 15, Hepes 10, Na2ATP 1 and EGTA 1 (250 ± 3 mosmol (kg H2O)−1; pH 7.3).
Single-channel recordings were carried out in the cell-attached mode, as described previously (Okada et al. 1994; Sabirov et al. 2000). A giga-seal was formed on cells which were pre-swollen in hypotonic high K+ solution. The hypotonic high K+ solution contained (mm): KCl 100, CaCl2 2, MgCl2 1, Hepes 5 and glucose 5. The pH was adjusted to 7.4 with KOH. The osmolality was 215 ± 3 mosmol (kg H2O)−1. The pipette solution was identical to the hypotonic bath solution used for whole-cell experiments.
Volume measurements and osmotic water permeability
The mean cell volume was measured at room temperature using a Coulter-type cell-sizing apparatus (CDA-500, Sysmex, Kobe, Japan), as reported previously (Hazama & Okada, 1988). Isotonic or hypotonic solution consisted of (mm): NaCl 95, KCl 4.5, MgCl2 1, CaCl2 1, mannitol 110 or 0 and Hepes 5 (pH 7.3, adjusted with NaOH; 310 or 200 mosmol (kg H2O)−1). Single-cell volume measurements were performed in spherical cells during the whole-cell patch-clamp recordings, as described previously (Morishima et al. 1998). Briefly, the cells were viewed with the transmitted light of an inverted microscope (Nikon, Tokyo, Japan) and imaged on a CCD camera (ICD-42AC, Ikegami, Tokyo, Japan). Data were recorded on videotape for subsequent off-line image analysis using a frame grabber, VISIONplus-AT (Image Technology, Inc., Bedford, MA, USA), controlled by an i-486-based computer (AST, USA). Images were collected every 5 s. The video system was calibrated with certified particle-size standards (glass microspheres, Duke Scientific Corporation, CA, USA). Osmotic water permeability, Pf, was calculated as reported previously (Sabirov et al. 1998), using the following relation:
| (1) |
where Vo is the initial cell volume, S is the cell surface area (cm2), Vw is the partial molar volume of water (18 cm3 mol−1), ΔΠ is the osmotic gradient and d(V/Vo)/dt is the rate of relative cell volume change.
Chemicals
All the reagents except for Na-Hepes (Nacalai Tesque, Kyoto, Japan), forskolin and EGTA (Wako, Osaka, Japan) were obtained from Calbiochem (San Diego, CA, USA). The stock solutions were prepared by dissolution in dimethyl sulfoxide (except for growth factors, see below), stored at −20 °C and diluted 1000 times in solutions just before use. The final DMSO concentration did not exceed 0.1 %. PDGF was reconstituted in 1 ml sterile 4 mm HCl containing 0.1 % bovine serum albumin and stored at −20 °C until use. EGF was dissolved in 10 mm Hepes buffer (pH 7.4). None of the vehicles affected the VSOR Cl− current at the concentrations used (≤ 0.1 %).
Data analysis
Data analysis was performed using WinASCD (kindly provided by G. Droogmans, KU Leuven), Clampfit (Axon Instruments) and Puls + Pulsfit (Heka Elektronik, Lambrecht, Germany) software packages. Statistical analysis, fittings and graphs were performed with Origin 6.0 or 6.1 (OriginLab Corporation, Northampton, MA, USA). To eliminate variations caused by differences in cell size, we have normalised current amplitudes by unit membrane capacitance. Values are means ± s.e.m. from n cells. Statistical significance was tested by Student's paired t test using P < 0.05 as the level of significance.
Experiments were performed at room temperature (23–25 °C).
RESULTS
Augmented activity of volume-sensitive Cl− channels in BPV-transfected cells
Mouse mammary C127 cells transfected with BPV alone or with BPV together with CFTR have been shown to respond to a hypotonic challenge with the activation of large Cl− currents associated with osmotic swelling, under the whole-cell configuration (Xia et al. 1996; Hazama et al. 2000). To examine the effects of BPV transfection, swelling-activated Cl− currents were recorded in control C127 cells and compared with those in BPV-transfected C127 cells in the present study. As shown in Fig. 1, both control C127 (control) and BPV-transfected C127 (BPV) cells exhibited activation of Cl− currents when swelling was induced by hypotonic stress (84 % osmolality). The half-maximal activation time was 475 ± 52 s (n = 7) in control cells and was not significantly different from that of 405 ± 37 s (n = 7) in BPV cells. The current in both cell types had the same phenotypic characteristics of VSOR Cl− current, such as outward rectification and time-dependent inactivation at large positive potentials. The two currents also shared sensitivity to phloretin (data not shown, n = 3), which was shown to cause a relatively specific block of VSOR Cl− currents in BPV/CFTR cells (Fan et al. 2001). However, the amplitude of peak current was 2.7 times greater in BPV cells than in control cells.
Figure 1. Effects of BPV transfection on whole-cell VSOR Cl− currents in C127 cells.
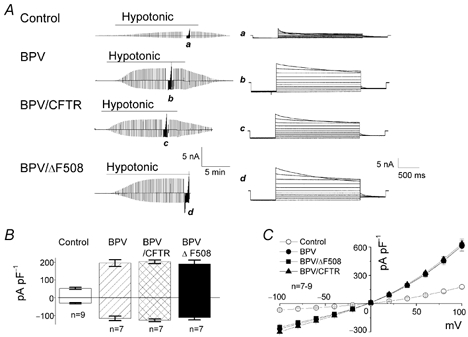
A, representative records for control (top panel), BPV (second panel), BPV/CFTR (third panel) and BPV/ΔF508 cells (bottom panel) before, during and after a hypotonic challenge. Alternating pulses from 0 to ±40 mV or step pulses from −100 to +100 mV in 20 mV increments (at a–d) were applied. Expanded traces of current responses to step pulses (a–d) are shown on the right. B, mean VSOR current densities recorded at ±40 mV in control, BPV, BPV/CFTR and BPV/ΔF508 cells after osmotic swelling under hypotonic conditions. The currents recorded from the three different BPV-transfected cells are significantly larger than that from the control. C, current–voltage relationships for instantaneous VSOR currents. Each symbol represents the mean instantaneous current density ± s.e.m. of 9, 7, 7 and 7 observations in control, BPV, BPV/CFTR and BPV/ΔF508 cells, respectively, under hypotonic conditions.
Similar VSOR Cl− currents were observed in BPV/CFTR and BPV/ΔF508 cells (Fig. 1). The half-maximal activation time was 345 ± 29 and 384 ± 70 s (n = 7) in BPV/CFTR and BPV/ΔF508 cells, respectively, and was not significantly different from that in BPV cells. The amplitude of peak currents (Fig. 1B) and the degree of outward rectification (Fig. 1C) were indistinguishable among the three types of BPV-transfected cells. We therefore conclude that BPV transfection induces upregulation of VSOR Cl− currents, irrespective of whether or not WT CFTR or the ΔF508 mutant is expressed.
Increased volume sensitivity of VSOR Cl− channel in BPV-transfected cells
Single Cl− channel currents could be consistently observed on both cell types when the patch pipette was attached to preswollen cells. Also, the channel activity quickly ran down after excision in ATP-free bath solution. These observations are in good accord with phenotypical properties of VSOR Cl− channel (Okada, 1997). Single-channel recordings in cell-attached patches showed time-dependent unitary closing events at large positive potentials in both swollen control and BPV/CFTR cells, as shown in Fig. 2A (four upper sets of traces). However, the channel stayed mostly in the open state and did not exhibit such time-dependent inactivation at +60 and +40 mV (Fig. 2A: two lower sets of traces) or at more negative potentials down to −100 mV (data not shown, n = 5–12), in both control and BPV-transfected cells.
Figure 2. Effects of BPV transfection on single VSOR Cl− channel conductance in C127 cells.
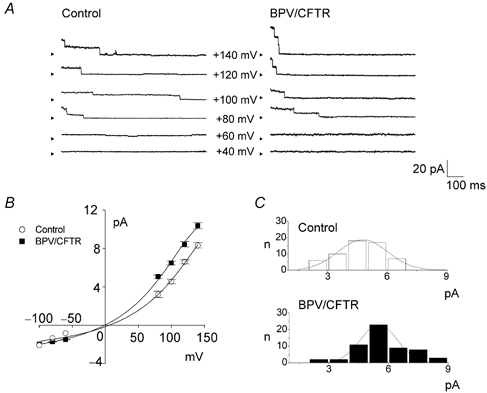
A, representative single-channel recordings from cell-attached patches on control (left panel) and BPV/CFTR cells (right panel), taken during application of step pulses (applied immediately before traces) from a holding potential of −140 mV to the indicated potentials (−Vp, where Vp is pipette potential). The number of active channels contained in a patch was 2.9 ± 0.4 and 3.8 ± 0.5 in control and BPV/CFTR cells, respectively. Arrowheads represent the zero current level. B, current–voltage relationships for the single-channel currents of control (○, n = 3–20) and BPV/CFTR cells (▪, n = 3–16). C, amplitude histogram plots for single-channel currents recorded at +100 mV from 58 control (upper panel) and 58 BPV/CFTR cells (lower panel) with s.d. values of 1.3 and 0.9, respectively.
Single-channel currents exhibited similar outward rectification in the two cell types (Fig. 2B). As shown in Fig. 2B and C, however, the single-channel conductance in BPV/CFTR cells (57.0 ± 1.7 pS at +100 mV, n = 58) was slightly larger compared with that in control cells (46.7 ± 1.4 pS at +100 mV, n = 58). However, this difference of 1.2 times in single-channel conductance can account for only a part of the large difference (of 2.7 times) between whole-cell current amplitudes of control and BPV-transfected cells. Since the open probability (Po) was around 1 at physiological potentials, BPV-induced enhancement of whole-cell VSOR Cl− conductance could be chiefly explained by an increase in the number of active channels.
The cell swelling rate did not significantly differ between control and BPV/CFTR cells, as shown in Fig. 3A. The osmotic water permeability (Pf) was essentially the same for the two cell types (inset, Fig. 3A). In contrast, when the current density was plotted as a function of the relative cell volume, which was measured simultaneously during whole-cell recordings, a striking difference between the two cell types was observed (Fig. 3B). As shown by the normalised curves (Fig. 3B, inset), the mid-point of activation was shifted to the left by BPV transfection. Volume sensitivity of the channel activity in BPV-transfected cells was much more prominent than in control cells. Thus, it is evident that the augmented activity of the VSOR Cl− channel can be ascribed to enhanced volume sensitivity of the channel.
Figure 3. Effects of BPV transfection on osmotic water permeability and volume expansion sensitivity of whole-cell VSOR Cl− currents in C127 cells.
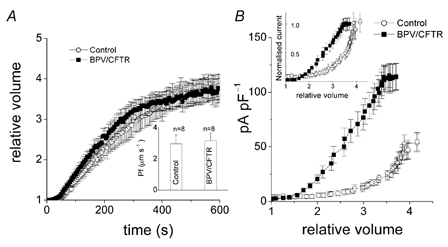
A, osmotic swelling rates in control (○, n = 8) and BPV/CFTR cells (▪, n = 8) under the whole-cell configuration. Cell volume was normalised to the volume before a hypotonic challenge. Osmotic water permeability (Pf) was calculated using eqn (1) and is summarised in the inset. B, whole-cell VSOR Cl− current densities in control (○, n = 8) and BPV/CFTR cells (▪, n = 8) plotted as a function of relative cell volume, which was monitored simultaneously during recordings. The inset shows the normalised current plotted against relative cell volume.
Activation of EGF receptors is associated with the augmentation of volume-sensitive Cl− conductance in BPV-transfected cells
Since BPV is known to contain a gene encoding the E5 protein, which induces constitutive activation of the PDGF receptor and EGF receptor in many cell types (Martin et al. 1989; Cohen et al. 1993; Nilson & DiMaio, 1993; Goldstein et al. 1994) including C127 cells (Petti et al. 1991; Petti & DiMaio, 1994), the effects of EGF and PDGF peptides on VSOR Cl− currents were studied in control and BPV cells. EGF and PDGF never affected the basal Cl− currents under isotonic conditions in control cells (data not shown, n = 3). The peptides were then applied after VSOR Cl− currents reached the steady-state level of full activation. As shown in Fig. 4A and C, EGF caused a further increase in VSOR Cl− current in control cells, but had no effect on the current in BPV cells. In contrast, PDGF failed to affect the Cl− current in either control or BPV-transfected cells (Fig. 4B and C).
Figure 4. Effects of EGF and PDGF peptides on whole-cell VSOR Cl− currents in control (left panel) and BPV-transfected C127 cells (right panel).
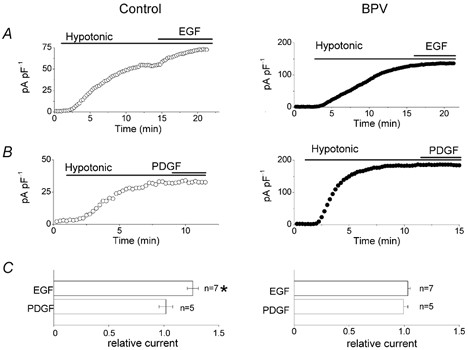
A and B, effects of EGF (50 ng ml−1) and PDGF (20 ng ml−1) on whole-cell VSOR current densities recorded at +40 mV in swollen control and BPV cells. C, summary of these effects. The current densities were normalised to the values recorded before application of EGF or PDGF. * Significantly different from the control values recorded before application of EGF or PDGF.
An inhibitor of EGF receptor tyrosine kinase-mediated phosphorylation, tyrphostin B46 (Gazit et al. 1991), suppressed VSOR Cl− currents in both control and BPV cells, as shown in Fig. 5A and B. The concentration– response curves (Fig. 5C) yield IC50 values of 31.4 ± 3.7 and 12.0 ± 0.9 μm (n = 4–5) for control and BPV cells, respectively. These data may suggest that BPV-transfected cells have a higher EGF receptor tyrosine kinase activity than control cells. In the presence of tyrphostin B46, swelling-induced activation of VSOR currents was almost completely prevented in control cells (data not shown, n = 6). In contrast, VSOR Cl− currents were not affected by an inhibitor of PDGF receptor tyrosine kinase, tyrphostin AG1296 (Kovalenko et al. 1994), in either control or BPV cells (Fig. 5A and B). These results suggest that the VSOR Cl− current in C127 cells is under the control of the EGF receptor, but not the PDGF receptor, signalling pathway.
Figure 5. Effects of EGF and PDGF receptor tyrosine kinase inhibitors on whole-cell VSOR Cl− currents in control and BPV-transfected C127 cells.
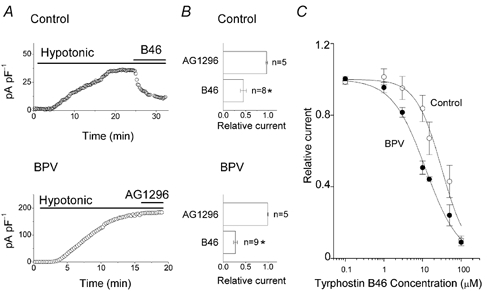
A, effects of the EGF receptor tyrosine kinase inhibitor tyrphostin B46 (50 μm) and the PDGF receptor tyrosine kinase inhibitor tyrphostin AG1296 (50 μm) on whole-cell VSOR Cl− currents recorded at +40 mV in swollen control (upper panel) and BPV cells (lower panel). B, summary of these effects. The current densities were normalised to the control values recorded before application of tyrphostin B46 or AG1296. * Significantly different from the control values. C, concentration–response curves for tyrphostin B46 effects in control (○, n = 4–5) and BPV cells (•, n = 4–5). Curves represent logistic non-linear curve fits. The IC50 and Hill coefficient were 31.7 ± 3.7 μm and 1.3 ± 0.2, respectively, in control cells and 12.0 ± 0.9 μm and 1.0 ± 0.1, respectively, in BPV cells.
During a hypotonic challenge (65 % osmolality), BPV cells were found to exhibit slow volume regulation after osmotic swelling (Fig. 6A, open circles), as was the case for BPV/CFTR cells (Hazama et al. 2000). The regulatory volume decrease (RVD) was not significantly affected by the presence of tyrphostin AG1296 (Fig. 6A, filled triangles). In contrast, tyrphostin B46 strongly impaired volume regulation after osmotic swelling (Fig. 6A, filled squares), suggesting the regulation of VSOR Cl− channels by EGF receptor tyrosine kinases in the RVD mechanism. In control C127 cells, RVD was also observed after osmotic swelling induced by a hypotonic challenge (Fig. 6B, open circles) though this was less prominent compared with BPV cells (Fig. 6A, open circles), as observed previously (Hazama et al. 2000). Tyrphostin B46 also exhibited an inhibitory effect on the RVD in control C127 cells (Fig. 6B, filled squares).
Figure 6. Effects of EGF and PDGF receptor tyrosine kinase inhibitors on volume regulation of BPV-transfected and control C127 cells after osmotic swelling.
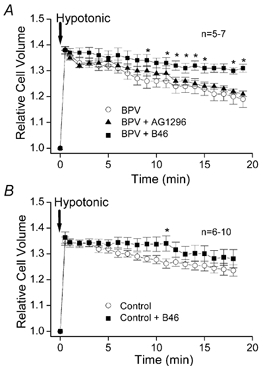
A hypotonic challenge was applied at time 0 to BPV-transfected (A) and control (B) cells in the absence of inhibitor (○), presence of 50 μm tyrphostin B46 (▪) or presence of 50 μm tyrphostin AG1296 (▴). Cell volume was normalised to the volume before a hypotonic challenge. Each symbol represents the mean of 5–7 observations. * Significantly different from the control values measured in the absence of tyrphostin B46 and AG1296.
DISCUSSION
The C127 cell line is derived from mouse mammary gland tumours (Lowy et al. 1978) and has been used as a recipient cell line for papilloma virus-induced transformation (Law et al. 1981; Reddy et al. 1987; Petti et al. 1991; Lai et al. 2000) or stable expression of various proteins, including CFTR (Marshall et al. 1994), using the entire BPV genome or its plasmid-containing part. In the present study, we investigated the effects of BPV-induced transformation on VSOR Cl− channels in this cell line.
Upregulation of VSOR Cl− channels due to constitutive activation of EGF receptor tyrosine kinase in BPV-transfected C127 cells
Transfection with the BPV genome confers a transformed phenotype on C127 cells (DiMaio et al. 2000). Indeed, BPV-transfected C127 cells, irrespective of whether or not WT or ΔF508 CFTR was coexpressed with BPV, exhibited a higher growth rate and a decreased contact inhibition compared with control C127 cells. In the present study, VSOR Cl− channel activity was found to be enhanced in BPV-transfected cells, irrespective of whether or not WT or ΔF508 CFTR was coexpressed (Fig. 1). This observation is in good agreement with previous reports that showed evidence for the involvement of the volume-sensitive Cl− channel in cell proliferation (Voets et al. 1995; Nilius et al. 1997b; Rouzaire-Dubois & Dubois, 1998; Wondergen et al. 2001).
Previous studies demonstrated that BPV E5, a 44 amino acid membrane-associated protein, leads to constitutive activation of tyrosine kinase-coupled receptors for EGF (Martin et al. 1989; Cohen et al. 1993) and PDGF (Petti et al. 1991; Cohen et al. 1993; Nilson & DiMaio, 1993; Goldstein et al. 1994; Petti & DiMaio, 1994; Lai et al. 2000) in many cell types. The following previous observations suggest EGF receptor involvement in the regulation of swelling-activated Cl− conductance: (1) EGF potentiated swelling-induced 125I− efflux from Intestine 407 cells (Tilly et al. 1993) and (2) a blocker of the EGF receptor tyrosine kinase, tyrphostin B46, suppressed volume-regulated Cl− currents in endothelial CPAE cells (Voets et al. 1998) and mouse fibroblasts (Bryan-Sisneros et al. 2000). In the present study, in fact, we found that EGF peptides enhanced VSOR Cl− currents in control C127 cells but did not further augment VSOR Cl− currents in BPV-transfected cells (Fig. 4). Also, tyrphostin B46 was found to inhibit VSOR Cl− currents more strongly in BPV-transfected cells than in control cells (Fig. 5). In contrast, PDGF peptides and an inhibitor of the PDGF receptor, tyrphostin AG2396, did not affect VSOR Cl− currents in either control or BPV-transfected C127 cells (Figs 4 and 5).
Enhancement of VSOR Cl− channel volume sensitivity with EGF receptor activation
Activation of the EGF receptor in response to an environmental stress such as irradiation or the presence of hydrogen peroxide, or by the binding of ligands including EGF and transforming growth factor TGFα, causes the generation of signals by the tyrosine-phosphorylated receptor protein. This enables the receptor to communicate with other membrane proteins, cytoskeletal proteins and the nucleus, and leads to cell proliferation, differentiation, migration and so on (Adamson & Wiley, 1997; Hubbard & Till, 2000). So far, it has been shown that EGF receptors regulate a number of cation channels, including Na+ channels (Greene & Tischler, 1982; Fanger et al. 1997; Hilborn et al. 1998), Ca2+ channels (Fu et al. 1997), K+ channels (Timpe & Fantl, 1994; Bowlby et al. 1997; Wischmeyer et al. 1998) and store-operated cation channels (Ma & Sansom, 2001). In the present study, we provide evidence for the EGF receptor-mediated regulation of anion channels.
The BPV E5 peptide has been shown to interact with the vacuolar H+ pump (Schapiro et al. 2000). Since intracellular pH is known to play a role in regulating VSOR Cl− channels (Nilius et al. 1998; Sakai et al. 1999; Sabirov et al. 2000), there is a possibility that intracellular pH (pHi) in BPV-transfected cells is different from that in control cells. However, 2′,7′-bis(carboxyethyl)-5-carboxyfluorescein (BCECF) fluorescence studies demonstrated that pHi values in control and BPV/CFTR cells were not significantly different from each other, under isotonic conditions as well as hypotonic conditions (K. Dezaki, I. F. Abdullaev, R. Z. Sabirov & Y. Okada, unpublished observations). Thus, it appears that augmented VSOR Cl− conductance in BPV-transfected cells cannot be accounted for by a difference in pHi.
Here it was observed that with activation of the EGF receptor tyrosine kinase, VSOR Cl− channel volume sensitivity was enhanced (Fig. 3). Augmentation of VSOR Cl− currents by stimulation of EGF receptors could be chiefly explained by an increase in the number of active channels. Thus, it is likely that activation of the EGF receptor facilitates swelling-induced channel transition from an off state to an open state, according to the gating model proposed by Jackson & Strange (1995). EGF receptor activation is known to lead to the activation of parallel signal transduction pathways, including those of phospholipase Cγ (PLCγ), phosphatidylinositol-3-kinase (PI3K) and a small guanine nucleotide-binding protein (Ras) as well as its downstream MAP kinase cascade (Schlessinger & Ullrich, 1992; Adamson & Wiley, 1997). However, we have not been able to identify the precise signalling pathway for EGF receptor-mediated upregulation of VSOR Cl− channels, because preliminary experiments (I. F. Abdullaev, R. Z. Sabirov & Y. Okada, unpublished observations) demonstrated that VSOR Cl− currents in C127 cells were not affected by application of the PLC inhibitor U73122 (50 μm), the PI3K inhibitor wortmannin (5 μm) or the MAPK/ERK kinase (MEK) inhibitor PD98059 (30 μm). The precise molecular mechanism of interactions between EGF receptors and volume-sensitive Cl− channels remains to be elucidated.
CFTR-mediated downregulation of VSOR Cl− channels does not occur in BPV-transfected cells
CFTR is known to be a multi-functional protein which acts as a Cl− channel per se and as a regulator for a number of channels and transporters (Schwiebert et al. 1999; Kunzelmann, 2001). Vennekens et al. (1999) reported CFTR-mediated downregulation of volume-regulated anion currents in CPAE and COS cells, when CFTR was expressed using the vector pCINeo/IRES-GFP. We reproduced this CFTR–VSOR Cl− channel interaction by expressing CFTR in HEK293T cells with the same expression vector (Ando-Akatsuka et al. 2002). In contrast, Xia et al. (1996) had previously reported that no evidence for an interaction between CFTR and swelling-activated Cl− channels could be found using BPV-transfected C127 cells either with or without coexpression of WT or ΔF508 CFTR. In the present study, VSOR Cl− currents were indeed found to be indistinguishable in BPV, BPV/CFTR and BPV/ΔF508 cells (Fig. 1). However, BPV transfection was seen to induce prominent augmentation of VSOR Cl− currents by constitutive activation of EGF receptors. This suggests that the CFTR–VSOR Cl− channel interaction was overridden by the activation of EGF receptor tyrosine kinase signalling pathways. In this regard, it is noteworthy that the CFTR protein itself may serve as a substrate of some tyrosine kinase (Fisher & Machen, 1996).
In conclusion, the present study demonstrates that the EGF receptor tyrosine kinase upregulates the activity of VSOR Cl− channels mainly by enhancing the volume sensitivity of active channels in murine mammary C127 cells.
Acknowledgments
We are grateful to J. Eggermont for providing the pCINeo/IRES-GFP plasmid, to H. Cheng for providing control, BPV and BPV/ΔF508 cells, to M. J. Welsh for providing BPV/CFTR cells and to S. Morishima for help with video-image analysis. We would like to express our appreciation to K. Dezaki and T. Kanaseki for helpful discussion; M. Ohara, E. L. Lee, S. Tanaka, K. Shigemoto, K. Manabe and C. Kondo for technical assistance; and T. Okayasu for secretarial assistance. This work was supported by Grant-in-Aid for Scientific Research (A) and for the Priority Area of ‘ABC Proteins’ from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a grant from the Salt Science Foundation.
References
- Abdullaev IF, Sabirov RZ, Ando-Akatsuka Y, Dezaki K, Okada Y. Effects of CFTR expression on volume-sensitive Cl− currents in HEK293T and C127 cells. Jpn J Physiol. 2001;51(suppl.):S173. [Google Scholar]
- Adamson ED, Wiley LM. The EGFR gene family in embryonic cell activities. Curr Top Dev Biol. 1997;35:71–120. doi: 10.1016/s0070-2153(08)60257-4. [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Abdullaev IF, Lee EL, Okada Y, Sabirov RZ. Down-regulation of volume-sensitive Cl− channels by CFTR is mediated by the second nucleotide-binding domain. Pflugers Arch. 2002;445:177–186. doi: 10.1007/s00424-002-0920-z. [DOI] [PubMed] [Google Scholar]
- Bowlby MR, Fadool DA, Holmes TC, Levitan IB. Modulation of the Kv1. 3 potassium channel by receptor tyrosine kinases. J Gen Physiol. 1997;110:601–610. doi: 10.1085/jgp.110.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan-Sisneros A, Sabanov V, Thoroed SM, Doroshenko P. Dual role of ATP in supporting volume-regulated chloride channels in mouse fibroblasts. Biochim Biophys Acta. 2000;1468:63–72. doi: 10.1016/s0005-2736(00)00243-1. [DOI] [PubMed] [Google Scholar]
- Cohen BD, Goldstein DJ, Rutledge L, Vass WC, Lowy DR, Schlegel R, Schiller JT. Transformation-specific interaction of the bovine papillomavirus E5 oncoprotein with the platelet-derived growth factor receptor transmembrane domain and the epidermal growth factor receptor cytoplasmic domain. J Virol. 1993;67:5303–5311. doi: 10.1128/jvi.67.9.5303-5311.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel V, Panenka W, Kelly ME, MacVicar BA. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J Neurosci. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio D, Lai CC, Mattoon D. The platelet-derived growth factor beta receptor as a target of the bovine papillomavirus E5 protein. Cytokine Growth Factor Rev. 2000;11:283–293. doi: 10.1016/s1359-6101(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Fan H-T, Morishima S, Kida H, Okada Y. Phloretin differentially inhibits volume-sensitive and cyclic AMP-activated, but not Ca-activated, Cl− channels. Br J Pharmacol. 2001;133:1096–1106. doi: 10.1038/sj.bjp.0704159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger GR, Vaillancourt RR, Heasley LE, Montmayeur JP, Johnson GL, Maue RA. Analysis of mutant platelet-derived growth factor receptors expressed in PC12 cells identifies signals governing sodium channel induction during neuronal differentiation. Mol Cell Biol. 1997;17:89–99. doi: 10.1128/mcb.17.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Machen TE. The tyrosine kinase p60c-src regulates the fast gate of the cystic fibrosis transmembrane conductance regulator chloride channel. Biophys J. 1996;71:3073–3082. doi: 10.1016/S0006-3495(96)79501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Scammell JG, Li M. Epidermal growth factor reduces L-type voltage-activated calcium current density in GH4C1 rat pituitary cells. Neuroendocrinol. 1997;65:157–163. doi: 10.1159/000127176. [DOI] [PubMed] [Google Scholar]
- Gazit A, Osherov N, Posner I, Yaish P, Poradosu E, Gilon C, Levitzki A. Tyrphostins. 2. Heterocyclic and alpha-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine kinases. J Med Chem. 1991;34:1896–1907. doi: 10.1021/jm00110a022. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Li W, Wang L-M, Heidaran MA, Aaronson S, Shinn R, Schlegel R, Pierce JH. The bovine papillomavirus type 1 E5 transforming protein specifically binds and activates the β-type receptor for the platelet-derived growth factor but not other related tyrosine kinase-containing receptors to induce cellular transformation. J Virol. 1994;68:4432–4441. doi: 10.1128/jvi.68.7.4432-4441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. PC12 pheochromocytoma cells in neurobiology research. Adv Cell Neurobiol. 1982;3:373–414. [Google Scholar]
- Hazama A, Fan H, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. Swelling-activated, cystic fibrosis transmembrane conductance regulator-augmented ATP release and Cl− conductances in C127 cells. J Physiol. 2000;523:1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama A, Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J Physiol. 1988;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn MD, Vaillancourt RR, Rane SG. Growth factor receptor tyrosine kinases acutely regulate neuronal sodium channels through the src signaling pathway. J Neurosci. 1998;18:590–600. doi: 10.1523/JNEUROSCI.18-02-00590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Single-channel properties of a volume-sensitive anion conductance. Current activation occurs by abrupt switching of closed channels to an open state. J Gen Physiol. 1995;105:643–660. doi: 10.1085/jgp.105.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko M, Gazit A, Bohmer A, Rorsman C, Ronnstrand L, Heldin C-H, Waltenberger J, Bohmer FD, Levitzki A. Selective platelet-derived growth factor receptor kinase blockers reverse sis-transformation. Cancer Res. 1994;54:6106–6114. [PubMed] [Google Scholar]
- Kubo M, Okada Y. Volume-regulatory Cl− channel currents in cultured human epithelial cells. J Physiol. 1992;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K. CFTR: interacting with everything? News Physiol Sci. 2001;16:167–170. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- Law MF, Lowy DR, Dvoretzky I, Howley PM. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981;78:2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Szabo I, Laun T, Kaba NK, Gulbins E, Lang F. The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes. J Cell Biol. 1998;141:281–286. doi: 10.1083/jcb.141.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Oiki S, Tsumura T, Shimizu T, Okada Y. Glibenclamide blocks volume-sensitive Cl− channels by dual mechanisms. Am J Physiol. 1998;273:C343–351. doi: 10.1152/ajpcell.1998.275.2.C343. [DOI] [PubMed] [Google Scholar]
- Lowy DR, Rands E, Scolnick EM. Helper-independent transformation by unintegrated harvey sarcoma virus DNA. J Virol. 1978;26:291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Sansom SC. Epidermal growth factor activates store-operated calcium channels in human glomerular mesangial cells. J Am Soc Nephrol. 2001;12:47–53. doi: 10.1681/ASN.V12147. [DOI] [PubMed] [Google Scholar]
- MacKenna DA, Dolfi F, Vuori K, Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101:301–310. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage due to disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci U S A. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, Fang S, Ostedgaard LS, O'Riordan CR, Ferrara D, Amara JF, Hoppe H, IV, Scheule RK, Welsh MJ, Smith AE. Stoichiometry of recombinant cystic fibrosis transmembrane conductance regulator in epithelial cells and its functional reconstitution into cells in vitro. J Biol Chem. 1994;269:2987–2995. [PubMed] [Google Scholar]
- Martin P, Vass WC, Schiller JT, Lowy DR, Velu TJ. The bovine papillomavirus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell. 1989;59:21–32. doi: 10.1016/0092-8674(89)90866-0. [DOI] [PubMed] [Google Scholar]
- Morishima S, Kida H, Ueda S, Chiba T, Okada Y. Water movement during cell volume regulation. In: Okada Y, editor. Cell Volume Regulation: The Molecular Mechanism and Volume Sensing Machinery. Amsterdam: Elsevier; 1998. pp. 209–212. [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyes G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997a;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Droogmans G. Modulation of volume-regulated anion channels by extra- and intracellular pH. Pflugers Arch. 1998;436:742–748. doi: 10.1007/s004240050697. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Kamouchi M, Viana F, Voets T, Droogmans G. Inhibition by mibefradil, a novel calcium channel antagonist, of Ca2+- and volume-activated Cl− channels in macrovascular endothelial cells. Br J Pharmacol. 1997b;121:547–555. doi: 10.1038/sj.bjp.0701140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson LA, Di Maio D. Platelet-derived growth factor receptor can mediate tumorigenic transformation by the bovine papillomavirus E5 protein. Mol Cell Biol. 1993;13:4137–4145. doi: 10.1128/mcb.13.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward rectifier Cl− channel: Fresh start to the molecular identity and volume sensor. Am J Physiol. 1997;273:C775–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Petersen CCH, Kubo M, Morishima S, Tominaga M. Osmotic swelling activates intermediate-conductance Cl− channels in human intestinal epithelial cells. Jpn J Physiol. 1994;44:403–409. doi: 10.2170/jjphysiol.44.403. [DOI] [PubMed] [Google Scholar]
- Petti L, Di Maio D. Specific interaction between the bovine papillomavirus E5 transforming protein and the β receptor for platelet-derived growth factor in stably transformed and acutely transfected cells. J Virol. 1994;68:3582–3592. doi: 10.1128/jvi.68.6.3582-3592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L, Nilson LA, Di Maio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991;10:845–855. doi: 10.1002/j.1460-2075.1991.tb08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VB, Garramone AJ, Sasak H, Wei CM, Watkins P, Galli J, Hsiung N. Expression of human uterine tissue-type plasminogen activator in mouse cells using BPV vectors. DNA. 1987;6:461–472. doi: 10.1089/dna.1987.6.461. [DOI] [PubMed] [Google Scholar]
- Rouzaire-Dubois B, Dubois JM. K+ channel block-induced mammalian neuroblastoma cell swelling: a possible mechanism to influence proliferation. J Physiol. 1998;510:93–102. doi: 10.1111/j.1469-7793.1998.093bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Morishima S, Okada Y. Probing the water permeability of ROMK1 and amphotericin B channels using Xenopus oocytes. Biochim Biophys Acta. 1998;1368:19–26. doi: 10.1016/s0005-2736(97)00176-4. [DOI] [PubMed] [Google Scholar]
- Sabirov RZ, Prenen J, Droogmans G, Nilius B. Extra- and intracellular proton-binding sites of volume-regulated anion channels. J Membr Biol. 2000;177:13–22. doi: 10.1007/s002320001090. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Qiu Z, Morgan JP, Izumo S. Tyrosine kinase activation is an immediate and essential step in hypotonic cell swelling-induced ERK activation and c-fos gene expression in cardiac myocytes. EMBO J. 1996;15:5535–5546. [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Nakamura F, Kuno M. Synergetic activation of outwardly rectifying Cl− currents by hypotonic stress and external Ca2+ in murine osteoclasts. J Physiol. 1999;515:157–168. doi: 10.1111/j.1469-7793.1999.157ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro F, Sparkowski J, Adduci A, Suprynowicz F, Schlegel R, Grinstein S. Golgi alkalinization by the papillomavirus E5 oncoprotein. J Cell Biol. 2000;148:305–315. doi: 10.1083/jcb.148.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J, Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev. 1999;79(suppl.):S145–S166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- Shen M-R, Chou C-Y, Browning JA, Wilkins RJ, Ellory JC. Human cervical cancer cells use Ca2+ signalling, protein tyrosine phosphorylation and MAP kinase in regulatory volume decrease. J Physiol. 2001;537:347–362. doi: 10.1111/j.1469-7793.2001.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M-R, Droogmans G, Eggermont J, Voets T, Ellory JC, Nilius B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J Physiol. 2000;529:385–394. doi: 10.1111/j.1469-7793.2000.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuba YM, Prevarskaya N, Lemonnier L, Van Coppenolle F, Kostyuk PG, Mauroy B, Skryma R. Volume-regulated chloride conductance in the LNCaP human prostate cancer cell line. Am J Physiol Cell Physiol. 2000;279:C1144–1154. doi: 10.1152/ajpcell.2000.279.4.C1144. [DOI] [PubMed] [Google Scholar]
- Sinning R, Schliess F, Kubitz R, Häussinger D. Osmosignalling in C6 glioma cells. FEBS Lett. 1997;400:163–167. doi: 10.1016/s0014-5793(96)01376-2. [DOI] [PubMed] [Google Scholar]
- Sorota S. Tyrosine protein kinase inhibitors prevent activation of cardiac swelling-induced chloride current. Pflugers Arch. 1995;431:178–185. doi: 10.1007/BF00410189. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Edixhoven MJ, Tertoolen LGJ, Morii N, Saitoh Y, Narumiya S, De Jonge HR. Activation of the osmo-sensitive chloride conductance involves P21rho and is accompanied by a transient reorganization of the F-actin cytoskeleton. Mol Biol Cell. 1996;7:1419–1427. doi: 10.1091/mbc.7.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly BC, Edixhoven MJ, Van Den Berghe N, Bot AGM, De Jonge HR. Ca2+-mobilizing hormones potentiate hypotonicity-induced activation of ionic conductances in Intestine 407 cells. Am J Physiol. 1994;267:C1271–1278. doi: 10.1152/ajpcell.1994.267.5.C1271. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Van Den Berghe N, Tertoolen LGJ, Edixhoven MJ, De Jonge HR. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J Biol Chem. 1993;268:19919–19922. [PubMed] [Google Scholar]
- Timpe LC, Fantl WJ. Modulation of a voltage-activated potassium channel by peptide growth factor receptors. J Neurosci. 1994;14:1195–1201. doi: 10.1523/JNEUROSCI.14-03-01195.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekens R, Trouet D, Vankeerberghen A, Voets T, Cuppens H, Eggermont J, Cassiman J-J, Droogmans G, Nilius B. Inhibition of volume-regulated anion channels by expression of the cystic fibrosis transmembrane conductance regulator. J Physiol. 1999;515:75–85. doi: 10.1111/j.1469-7793.1999.075ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. J Physiol. 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Szücs G, Droogmans G, Nilius B. Blockers of volume-activated Cl− currents inhibit endothelial cell proliferation. Pflugers Arch. 1995;431:132–134. doi: 10.1007/BF00374387. [DOI] [PubMed] [Google Scholar]
- Wischmeyer E, Doring F, Karschin A. Acute suppression of inwardly rectifying Kir2 channels by direct tyrosine kinase phosphorylation. J Biol Chem. 1998;273:34063–34068. doi: 10.1074/jbc.273.51.34063. [DOI] [PubMed] [Google Scholar]
- Wondergem R, Gong W, Monen SH, Dooley SN, Gonce JL, Conner TD, Houser M, Ecay TW, Ferslew KE. Blocking swelling-activated chloride current inhibits mouse liver cell proliferation. J Physiol. 2001;532:661–672. doi: 10.1111/j.1469-7793.2001.0661e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Krouse ME, Fang RH, Wine JJ. Swelling and Ca2+-activated anion conductances in C127 epithelial cells expressing WT and ΔF508-CFTR. J Membr Biol. 1996;151:269–278. doi: 10.1007/s002329900077. [DOI] [PubMed] [Google Scholar]


