Abstract
The physiological importance of CD98 surface antigen in regulating placental trophoblast cell fusion and amino acid transport activity has been studied in parallel in a cell model of syncytialization (the cytotrophoblast cell line BeWo following increased intracellular cAMP by forskolin treatment) using antisense oligonucleotides. CD98 protein abundance (determined by Western blot) was decreased by 50 % following antisense oligonucleotide transfection. Transfection with antisense oligonucleotide altered the responses of BeWo to forskolin. Cell fusion (determined by a quantitative flow cytometry assay) was inhibited by 57 %, and both human chorionic gonadotropin secretion and L-leucine influx through system L were suppressed. These findings show that CD98 is involved in the process of cell fusion necessary for syncytiotrophoblast formation and that during this physiologically important event, amino acid transport activity is also regulated through expression of this membrane protein.
The trophoblast surface of the human placenta, in direct contact with maternal blood, forms a syncytium (the syncytiotrophoblast), which is derived from the underlying cell layer, the cytotrophoblast. Syncytialization is an unusual biological process that occurs in only a few human cell lineages (for example in the development of myotubes, osteoclasts and syncytiotrophoblasts), and its mechanism remains to be elucidated. Two human endogenous retroviral proteins (HERV-3 and HERV-W) have been found to be relevant to human trophoblast syncytialization. HERV-3 is an endogenous retroviral gene product expressed in syncytiotrophoblasts, and circumstantial evidence suggests that it may play a role in cytotrophoblast differentiation (Lin et al. 2000). HERV-W encodes the membrane protein syncytin (Mi et al. 2000), the viral homologue of which, ERV-W, is also highly fusogenic, inducing syncytium formation upon interaction with the type D mammalian retrovirus receptor (Blond et al. 2000), the amino acid transporter B0 (ASCT2) (Tailor et al. 1999; Lavillette et al. 2002).
CD98 cell surface antigen is an integral membrane protein (Devés & Boyd, 2000), which is expressed on the cytotrophoblast and particularly on the plasma membrane of the syncytiotrophoblast (demonstrated functionally by Ayuk et al. (2000) and immunohistochemically by Okamoto et al. (2002)). This molecule has been found to be identical to fusion regulatory protein-1 (FRP-1) and its expression is necessary for virus-induced cell fusion (Tsurudome & Ito, 2000) and for osteoclast formation (Tajima et al. 1999). Importantly, CD98 is also able to activate specific integrins and thus may be of particular importance in cell adhesion (Fenczik et al. 1997; Takahashi et al. 2001). CD98 is thus a multifunctional protein that, like the syncytin receptor, is also involved in amino acid transport; the heavy chain forms part of a dimeric structure with one of a family of light chains (Wagner et al. 2001). In recent experiments using cDNA microarrays (Kudo et al. 2002b) we found a time-dependent increase in the expression of CD98 mRNA during syncytialization of BeWo cells following forskolin treatment (Y. Kudo, C. A. R. Boyd, T. C. Freeman, I. L. Sargent & C. W. G. Redman, unpublished observations). We have now studied the importance of CD98 both to the process of cell fusion and to amino acid transport function during placental trophoblast syncytialization by manipulating the level of protein expression using transient transfection with antisense oligonucleotides to this molecule. We have used BeWo cells as a well-established cellular model of placental trophoblast syncytialization (Ringler & Strauss, 1990).
METHODS
BeWocell culture
Cloned BeWo cells expressing either a fusion protein of green fluorescent protein and human histone H2B (H2B-GFP) or a fusion protein of red fluorescent protein and the mitochondrial targeting sequence from subunit VIII of human cytochrome C oxidase (Mit-DsRed2) were maintained at 37 °C as monolayers in Ham's F-12 medium supplemented with 10 % fetal bovine serum, 2 mM L-glutamine, 100 u ml−1 penicillin and 100 u ml−1 streptomycin in a humidified atmosphere of 5 % CO2 and 95 % air (Kudo et al. 2002a). These two cell lines were subcultured by treating with 0.05 % trypsin in Ca2+- and Mg2+-free phosphate buffered saline (PBS) containing 0.02 % ethylenediamine-tetraacetate, then mixed in equal numbers and seeded into 35 mm plastic culture dishes and grown for 2–3 days to the stage of 50 % confluency. At 50 % confluency, 3 μg per well antisense oligonucleotide to human CD98 (5′-CCTGGCTCATGGTGCCTG-3′) (Chen et al. 1996) or mismatch oligonucleotide (5′-GGTCCCTCATCCTGGGTG-3′) was introduced into cells using FuGENE 6 transfection reagent according to the manufacturer's protocol by incubating cells for 12 h. Forskolin or vehicle (dimethyl sulphoxide) was then added to a final concentration of 100 μM and cultures were further incubated at 37 °C for the indicated times. At the end of the incubation period, cells were more than 95 % viable as assessed by trypan blue dye exclusion. Cultures were conducted in triplicate for each set of experiments to assess reproducibility. The conditioned medium was collected and centrifuged at 3000 g at 4 °C for 10 min to remove cellular debris and stored at −70 °C until use.
Cell fusion analysis
The rate of cell fusion (syncytialization) was measured by flow cytometry, as described previously (Kudo et al. 2002a, 2003). Cells were harvested by trypsinization and fixed, and the number of single fluorescent (green or red) positive cells (i.e. non-fused or non-detectably fused cells) and double fluorescent (green and red) positive cells (i.e. detectably fused cells) were counted using a flow cytometer (EPICS Altra, Beckman Coulter, High Wycombe, UK). Twenty thousand cells were analysed for each sample.
Human chorionic gonadotropin (hCG) secretion
hCG secretion was determined by measuring its concentration in the conditioned medium using an immunoassay kit that specifically detects the β-chain of hCG.
Western blot
Cells were harvested by trypsinization, washed twice with ice-cold PBS, suspended in 1 ml of ice-cold PBS containing 50 μl (g tissue)−1 protease inhibitor mixture and disrupted by sonication at a power of 25 W for 30 s whilst held in an ice bath. The homogenate was centrifuged at 15 000 g for 15 min at 4 °C. The resultant supernatant (cellular extract) was stored at −70 °C until use. The cellular extracts were mixed with Laemmli sample buffer (Laemmli, 1970) and boiled for 5 min before loading. Samples (20 μg protein per lane) were separated by electrophoresis under reducing conditions on 12 % (w/v) sodium dodecyl sulphate-polyacrylamide gels and transferred to nitrocellulose membrane. After blocking by incubation in Tris buffered saline (TBS) containing 2 % (w/v) bovine serum albumin (BSA) for 1 h at room temperature, the membrane was soaked overnight at 4 °C in TBS containing CD98 polyclonal antibody (1:200 dilution) and 1 % (w/v) BSA. The membrane was rinsed and washed three times for 5 min each in TBS containing 0.1 % (v/v) Tween-20 (TBS-T), incubated with peroxidase-linked anti-rabbit IgG antibody (1:5000 dilution) in TBS-T for 1 h at room temperature and then rinsed and washed three times for 5 min each in TBS-T followed by one wash in TBS for 5 min. Proteins were detected with the ECL detection system. The intensity of the band for each sample was quantified using an image documentation and analysis system (GDS8000, Ultra-Violet Products, Cambridge, UK).
Amino acid influx studies
After aspirating culture medium, each dish was washed twice with pre-warmed (37 °C) PBS and cells were depleted of intracellular amino acids by incubating in PBS at 37 °C for 30 min to minimize any trans effects. The influx of amino acid was initiated by replacing the PBS with pre-warmed Na+-free PBS containing 2 μM L-[3H]leucine, followed by further incubation at 37 °C. Na+-free PBS was prepared by replacing NaCl, NaHCO3 and NaH2PO4 with choline chloride, choline bicarbonate and KH2PO4, respectively. Other additions are described in the figure legends. Following aspiration of isotope solution, cells were quickly washed with ice-cold PBS containing 10 mM unlabelled L-leucine. Then 0.1 M NaOH and 0.1 % sodium dodecyl sulphate solution were added for solubilization and aliquots were taken for liquid scintillation counting and protein determination.
Immunocytochemistry
Cryostat sections of term human placenta (10 μm thick) were permeabilized with 50 % then 70 % ethanol on Vectabond coated slides. To minimize non-specific staining, they were then incubated with 10 % goat serum in PBS containing 2 % BSA for 10 min. Sections were incubated overnight at 4 °C with 1:200 polyclonal rabbit anti-human CD98 (sc-9160; Santa Cruz Biotechnology, Inc.) in PBS containing 2 % BSA. Following three 10 min washes in PBS, sections were incubated for 1 h at room temperature with FITC-labelled goat anti-rabbit IgG (F0382; Sigma) diluted 1:200 in PBS containing 2 % BSA. Following three further washes (10 min each) in PBS, sections were wet-mounted with Vectashield. These were used to determine the cellular localization of CD98hc immunoreactivity in chorionic villi by confocal fluorescence microscopy (Hussain et al. 2002). Control sections, from which either primary or secondary antibody was omitted, showed no cellular fluorescence.
Protein estimation
Protein concentration of the cell extract was determined by the method of Lowry et al. (1951), using bovine serum albumin as a standard.
Statistical analysis
Differences between groups were analysed using ANOVA and results were considered statistically significant at P < 0.05.
Materials
BeWo cells (passage number approximately 40) were kindly given by Dr S. L. Greenwood (Academic Unit of Child Health, St. Mary's Hospital, University of Manchester, UK). L-[4,5-3H]Leucine (53.0 Ci mmol−1 or 1.96 TBq mmol−1), peroxidase-linked anti-rabbit IgG antibody and the ECL detection system were purchased from Amersham Life Sciences (Amersham, Little Chalfont, UK). CD98 (H-300) rabbit polyclonal antibody raised against a human recombinant protein was obtained from Autogen Bioclear (Calne, Wiltshire, UK). Forskolin, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) and tissue protease inhibitor mixture were obtained from Sigma-Aldrich Chemical (Poole, UK), FuGENE 6 was from Roche Diagnostics Ltd (Lewes, UK), tissue culture supplements were from Gibco BRL (Paisley, UK) and the human chorionic gonadotropin (hCG) chemiluminescent immunoassay kit was from Euro/DPC (Llanberis, UK). All chemicals were of the highest purity commercially available.
RESULTS
In order to determine the effect on the rate of cell fusion of transfection with antisense oligonucleotide to CD98, antisense or mismatch oligonucleotide was introduced into cells. Cells were then cultured in the presence or absence of forskolin and the number of fused cells was counted (Fig. 1). Cell fusion was stimulated in a time-dependent manner by the presence of forskolin, peaked at 48 h after the initiation of forskolin treatment and declined thereafter. In cells transfected with antisense oligonucleotide, the number of fused cells initially increased (up to 12 h) following forskolin treatment, but no significant increase was observed thereafter, such that after 48 h culture the number of fused cells was approximately 50 % of that in untransfected cells or in cells transfected with mismatch oligonucleotide. Between 12 and 24 h the rate of syncytialization was decreased 3-fold and between 24 and 48 h it was decreased 5.7-fold by antisense oligonucleotide transfection. Forskolin-induced hCG secretion, a marker of syncytialization in BeWo cells, was suppressed in cells transfected with antisense oligonucleotide compared to untreated cells or cells transfected with mismatch oligonucleotide (Table 1).
Figure 1. Effect on cell fusion of antisense oligonucleotide to CD98.
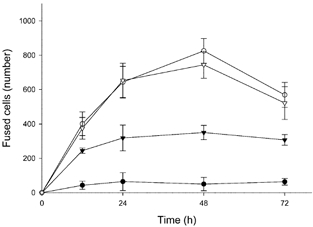
BeWo cells expressing either H2B-GFP or Mit-DsRed2 were mixed, cultured and transfected without (•, ○) or with antisense (▾) or mismatch (▿) oligonucleotide. Cells were further cultured with 100 μM forskolin (○, ▾, ▿) or vehicle (•) for the time indicated. Cell fusion was analysed on a flow cytometer, as described in Methods. Data represent the means ± S.D. of three separate experiments.
Table 1.
Effect of antiense olioneuleotide CD98 on hCG secretion in BeWo cells
| Treatment | hCG (mi.u.ml−1) |
|---|---|
| Control | 94.7 ± 6.8 |
| Vehicle | 614.7 ± 76.8* |
| Forskolin | 6369.7 ± 390.7*† |
| Forskolin + antisense | 3641.7 ± 232.3*†‡ |
| Forskolin + mismatch | 6233.7 ± 537.6*† |
Significantly different from control.
Significantly different from values for cells cultured with vehicle alone.
Significantly different from values for cells cultured with forskolin alone. BeWo cells expressing either H2B-GFP or Mit-DsRed2 were mixed, cultured and transfected with or without antisense or mismatch oligonucleotide. hCG concentrations in the conditioned medium before (control) and after further 48 h treatment with 100 μM forskolin or vehicle were analysed as described in Methods. Values are means ±s.d. of three separate experiments with triplicate assays.
Figure 2 shows Western blot analysis of CD98 protein levels in extracts of control cells and of cells transfected with antisense or mismatch oligonucleotide followed by 48 h forskolin treatment. As expected under reducing conditions (Okamoto et al. 2002), a band of 75–80 kDa was found. In cells not transfected with antisense oligonucleotide, forskolin addition produced a marked (2.6-fold) stimulation in CD98 protein levels compared with those before treatment or after treatment with vehicle. Antisense oligonucleotide transfection before forskolin treatment significantly suppressed forskolin-induced stimulation of CD98 protein levels. In contrast, mismatch oligonucleotide showed no significant effect on forskolin-induced stimulation of CD98 protein quantity.
Figure 2. Effect on its protein expression level of antisense oligonucleotide to CD98.
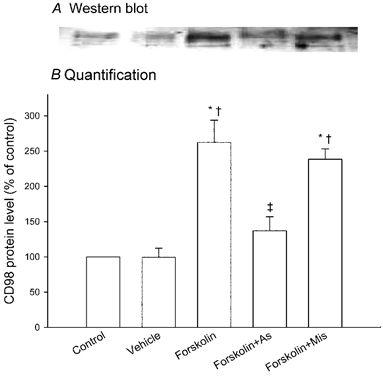
BeWo cells expressing either H2B-GFP or Mit-DsRed2 were mixed, cultured and transfected without or with antisense or mismatch oligonucleotide. CD98 protein levels in the cellular extracts before (control) and after a further 48 h treatment with 100 μM forskolin or with vehicle were analysed as described in Methods. A, Western blot (under reducing conditions). The results presented are from a single representative experiment. B, quantification of CD98 protein levels. The intensity of each band was quantified using an image documentation and analysis system. Data represent the means ± S.D. of three separate experiments, expressed as percentage of control (i.e. values without culture). * Significantly different from control. † Significantly different from values for cells cultured with vehicle alone. ‡ Significantly different from values for cells cultured with forskolin alone. As, antisense oligonucleotide; Mis, mismatch oligonucleotide.
Figure 3A compares the influx of 2 μM L-leucine into BeWo cells (transfected with or without antisense or mismatch oligonucleotide) before and after forskolin treatment, under initial rate conditions in the absence of Na+. The data show carrier-mediated influx, defined by subtracting the diffusional component from the total influx. The diffusional component was determined by measuring the influx of 2 μM L-[3H]leucine in the presence of 20 mM unlabelled L-leucine. In cells not transfected with antisense oligonucleotide, total carrier-mediated influx was increased 1.6-fold by culture for 48 h in the presence of forskolin, compared with that before culture or after culture with vehicle. This stimulation was suppressed significantly in antisense oligonucleotide-transfected cells. Transfection with mismatch oligonucleotide had no significant effect on L-leucine influx. In order to separate the transport pathways contributing to total Na+-independent flux, the synthetic amino acid 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) (Christensen, 1979), a system L-specific analogue, or unlabelled L-leucine was added at a concentration of 2 mM. The BCH-inhibitable component (i.e. system L-mediated influx) was enhanced following forskolin treatment. In antisense, but not in mismatch, oligonucleotide-transfected cells, this system L-mediated flux was significantly suppressed (Fig. 3B).
Figure 3. Effect on L-leucine influx of antisense oligonucleotide to CD98.
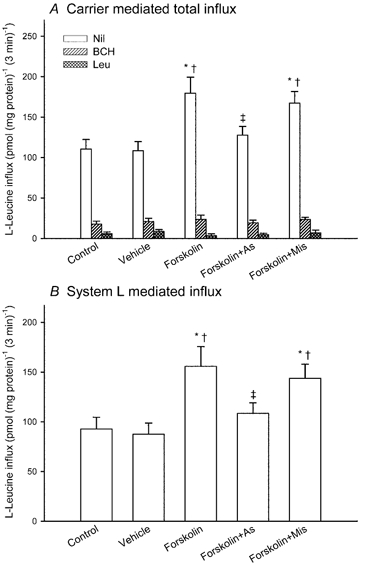
BeWo cells expressing either H2B-GFP or Mit-DsRed2 were mixed, cultured and transfected without or with antisense or mismatch oligonucleotide. L-Leucine influx was measured over a 3 min period before treatment (control) and after a further 48 h treatment with 100 μM forskolin or with vehicle in a medium containing 2 μM L-[ H]leucine with or without 2 mM 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) or 2 mM L-leucine in the absence of Na+. A, carrier-mediated influx rate defined by subtracting the diffusional component (determined by measuring the influx of 2 μM L-[3H]leucine in the presence of 20 mM unlabelled L-leucine) from the total influx. B, system L-mediated influx rate defined as the difference between the influx in the presence and absence of BCH. Data represent the means ± S.D. of three separate experiments of triplicate assays. * Significantly different from control. † Significantly different from values for cells cultured with vehicle alone. ‡ Significantly different from values for cells cultured with forskolin alone. As, antisense oligonucleotide; Mis, mismatch oligonucleotide.
Figure 4 shows localization of immunoreactive CD98 heavy chain in chorionic villi from term human placenta. Note that both the syncytiotrophoblast and, where present, underlying cytotrophoblast cells are immunoreactive. In syncytiotrophoblast, the expression of CD98 immunoreactivity is particularly marked in the basal membrane.
Figure 4. Confocal microscopy of human term placenta showing immunofluorescence localization of CD98.
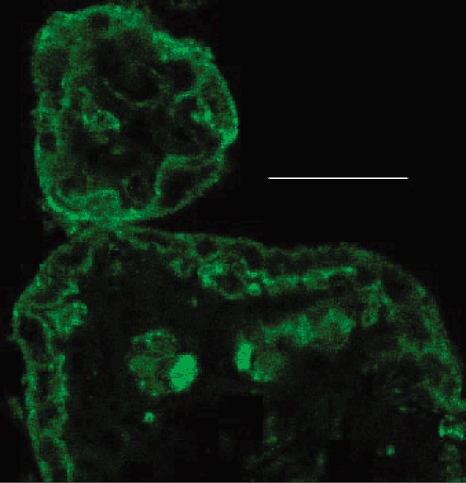
The section shows two adjacent chorionic villi cut transversely. There is positive immunostaining of (syncytio- and cyto-) trophoblast, as well as of components in the villus core (fetal capillary endothelium; fetal cells present within the capillary lumen). Scale bar represents 10 μm.
DISCUSSION
We conducted the experiments described here to ask questions concerning the functional role of CD98 in placental syncytiotrophoblast formation. For experimental reasons we have used a model human choriocarcinoma cell line, which is considered to have many of the properties of freshly isolated human trophoblast (Ringler & Strauss, 1990). We found experimental evidence that CD98 is involved in cell fusion and that its expression level correlates with amino acid transport activity through system L in this type of cell. This conclusion arises from the following observations; (1) CD98 protein level is increased following cell fusion; (2) when CD98 protein expression is inhibited by transfection with antisense oligonucleotide, the rate of cell fusion and hCG secretion are both suppressed; (3) amino acid influx through system L is enhanced following fusion; (4) this enhanced system L activity is inhibited by antisense oligonucleotide to CD98; and (5) mismatch oligonucleotide to CD98 has no significant effect on forskolin-induced stimulation of cell fusion, hCG secretion or amino acid influx through system L.
During placental development cytotrophoblasts differentiate along two pathways. In one, cytotrophoblasts (extravillous cytotrophoblasts) invade the uterine wall and its blood vessels, which supply oxygenated maternal blood to the intervillous space. The involvement of certain extracellular matrix components and adhesion molecules, including the integrin family, in this process, both during physiological and pathological development, has been extensively studied (Genbacev et al. 1997; Zhou et al. 1998; MacPhee et al. 2001). In the other pathway, the focus of this study, cytotrophoblasts fuse with each other to generate multinucleated syncytiotrophoblasts. Although the mechanism of this process is still poorly understood, the results described here suggest that CD98 expression is indeed involved. Using immunocytochemistry, Okamoto et al. (2002) have shown that the CD98 protein is expressed at the surface of human trophoblast; this is confirmed by the data shown in Fig. 4. However we find, using a different primary antibody, that CD98 immunoreactivity is prominent particularly at the basal surface (in direct contact with the underlying cytotrophoblast) of the trophoblast of the term human placenta (see also Kudo & Boyd, 2002). The fact that CD98 immunoreactivity is detected in human chorionic villus trophoblast in situ as well as in a choriocarcinoma cell line (BeWo) in vitro (Fig. 2) suggests that the functional results in the BeWo model system described here will have relevance to understanding syncytialization in normal placental development. The mechanism of CD98-induced cell fusion may be via integrin activation (Fenczik et al. 1997; Miyamoto et al. 2003). It is therefore an intriguing possibility that CD98 expression may set the pathway of cytotrophoblast differentiation (i.e. invasion or fusion) during early placental development.
CD98, as a common heavy chain, forms heterodimers with the family of light chains (system L-amino acid transporter-1 (LAT-1), system L-amino acid transporter-2 (LAT-2), system y+ L-amino acid transporter-1 (y+ LAT-1), system y+ L-amino acid transporter-2 (y+ LAT-2) and system XC−-amino acid transporter (xCT)) and is required for amino acid transport function by this family of heterodimeric molecules. Mastroberardino and colleagues (1998) have proposed that in Xenopus oocytes it is expression of the heavy chain that is necessary for maturation, transport and/or surface residence of heterologously expressed light chain subunits; in epithelia an additional role of the heavy chain may be to determine the polarity of transporter expression (Pfeiffer et al. 1999; Pineda et al. 1999). We previously demonstrated that it is CD98 heavy chain that is rate limiting for systems L and y+ L activity, using a placental villous explant system (Kudo & Boyd, 2000). In the present study we manipulated CD98 expression by transfection with antisense oligonucleotide and found that CD98 protein level correlates with system L activity. For system y+ L, Fei and colleagues (1995) showed that following antisense depletion of the heavy chain of CD98 from total placental mRNA there was no longer expression of this transport function. Our analysis using cDNA microarray of forskolin-induced BeWo cell syncytialization (Kudo et al. 2002b) showed that there are also individual specific changes in the expression of the light chains (LAT-1 and LAT-2 are increased time dependently, y+ LAT-1 transiently decreased and y+ LAT-2 transiently increased) (Y. Kudo, C. A. R. Boyd, T. C. Freeman, I. L. Sargent & C. W. G. Redman, unpublished observations). The precise mechanisms for activation of amino acid influx supported by heterodimeric transporters, as observed in the present study, will require future studies aimed at determining protein abundances, and the subcellular localizations and stabilities of each of these proteins as well as mRNA, as have been done in T-cells (Nii et al. 2001).
Acknowledgments
We thank Dr R. Branton for the FACS analysis and Mrs J. Bellinger for carrying out the hCG assays. We are grateful to the EP Abraham Trust, Sir William Dunn School of Pathology, University of Oxford for financial support.
REFERENCES
- Ayuk PT, Sibley CP, Donnai P, D'Souza S, Glazier JD. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278:C1162–1171. doi: 10.1152/ajpcell.2000.278.6.C1162. [DOI] [PubMed] [Google Scholar]
- Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel Fernandes S, Mandrand B, Mallet F, Cosset FL. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Meredith D, Boyd CAR. Both the H13 gene product and 4F2 antigen are involved in the induction of system y+ cationic amino-acid transport following activation of human peripheral blood mononuclear cells (PBM) Biochim Biophys Acta. 1996;1284:1–3. doi: 10.1016/0005-2736(96)00125-3. [DOI] [PubMed] [Google Scholar]
- Christensen HN. Exploiting amino acid structure to learn about membrane transport. Adv Enzymol. 1979;49:41–101. doi: 10.1002/9780470122945.ch2. [DOI] [PubMed] [Google Scholar]
- Devés R, Boyd CAR. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J Memb Biol. 2000;173:165–177. doi: 10.1007/s002320001017. [DOI] [PubMed] [Google Scholar]
- Fei YJ, Prasad PD, Leibach FH, Ganapathy V. The amino acid transport system y+L induced in Xenopus laevis oocytes by human choriocarcinoma cell (JAR) mRNA is functionally related to the heavy chain of the 4F2 cell surface antigen. Biochemistry. 1995;34:8744–8751. doi: 10.1021/bi00027a025. [DOI] [PubMed] [Google Scholar]
- Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81–85. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Hussain I, Kellett GL, Affleck J, Shepherd J, Boyd CAR. Expression and cellular distribution during development of the peptide transporter (PepT1) in the small intestinal epithelium of the rat. Cell Tiss Res. 2002;307:139–142. doi: 10.1007/s00441-001-0473-z. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. Heterodimeric amino acid transporters: expression of heavy but not light chains of CD98 correlates with induction of amino acid transport systems in human placental trophoblast. J Physiol. 2000;523:13–18. doi: 10.1111/j.1469-7793.2000.t01-1-00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. Human placental amino acid transporter genes: expression and function. Reproduction. 2002;124:593–600. doi: 10.1530/rep.0.1240593. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR, Kimura H, Cook PR, Redman CWG, Sargent IL. Quantifying the syncytialisation of human placental trophoblast BeWo cells grown in vitro. Biochim Biophys Acta. 2003;1640:25–31. doi: 10.1016/s0167-4889(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR, Kimura H, Cook PR, Sargent IL, Redman CWG. Quantifying the syncytialisation of a human placental trophoblast cell line grown in vitro. J Physiol. 2002a;543.P:57P. doi: 10.1016/s0167-4889(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR, Sargent IL, Redman CWG, Stephens R, Scott L, Freeman TC. Microarray analysis of human placental cytotrophoblast (BeWo cell) syncytialisation induced by forskolin. J Physiol. 2002b;539.P:127P. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset FL, Kabat D. The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J Virol. 2002;76:6442–6452. doi: 10.1128/JVI.76.13.6442-6452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Xu B, Rote NS. The cellular mechanism by which the human endogenous retrovirus ERV-3 env gene affects proliferation and differentiation in a human placental trophoblast model, BeWo. Placenta. 2000;21:73–78. doi: 10.1053/plac.1999.0443. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MacPhee DJ, Mostachfi H, Han R, Lye SJ, Post M, Caniggia I. Focal adhesion kinase is a key mediator of human trophoblast development. Lab Invest. 2001;81:1469–1483. doi: 10.1038/labinvest.3780362. [DOI] [PubMed] [Google Scholar]
- Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, Lavallie E, Tang XY, Edouard P, Howes S, Keith JC, Jr, McCoy JM. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- Miyamoto YJ, Mitchell JS, McIntyre BW. Physical association and functional interaction between beta1 integrin and CD98 on human T lymphocytes. Mol Immunol. 2003;39:739–751. doi: 10.1016/s0161-5890(02)00255-9. [DOI] [PubMed] [Google Scholar]
- Nii T, Segawa H, Taketani Y, Tani Y, Ohkido M, Kishida S, Ito M, Endou H, Kanai Y, Takeda E, Miyamoto K. Molecular events involved in up-regulating human Na+-independent neutral amino acid transporter LAT1 during T-cell activation. Biochem J. 2001;358:693–704. doi: 10.1042/0264-6021:3580693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Sakata M, Ogura K, Yamamoto T, Yamaguchi M, Tasaka K, Kurachi H, Tsurudome M, Murata Y. Expression and regulation of 4F2hc and hLAT1 in human trophoblasts. Am J Physiol Cell Physiol. 2002;282:C196–204. doi: 10.1152/ajpcell.2002.282.1.C196. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R, Rossier G, Spindler B, Meier C, Kuhn L, Verrey F. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J. 1999;18:49–57. doi: 10.1093/emboj/18.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- Ringler GE, Strauss JF., 3rd In vitro systems for the study of human placental endocrine function. Endo Rev. 1990;11:105–123. doi: 10.1210/edrv-11-1-105. [DOI] [PubMed] [Google Scholar]
- Tailor CS, Nouri A, Zhao Y, Takeuchi Y, Kabat D. A sodium-dependent neutral-amino-acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J Virol. 1999;73:4470–4474. doi: 10.1128/jvi.73.5.4470-4474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M, Higuchi S, Higuchi Y, Miyamoto N, Uchida A, Ito M, Nishio M, Komada H, Kawano M, Kusagawa S, Tsurudome M, Ito Y. Suppression of FRP-1/CD98-mediated multinucleated giant cell and osteoclast formation by an anti-FRP-1/CD98 mAb, HBJ 127, that inhibits c-src expression. Cell Immunol. 1999;193:162–169. doi: 10.1006/cimm.1999.1467. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Bigler D, Ito Y, White JM. Sequence-specific interaction between the disintegrin domain of mouse ADAM 3 and murine eggs: role of beta1 integrin-associated proteins CD9, CD81, and CD98. Mol Biol Cell. 2001;12:809–820. doi: 10.1091/mbc.12.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurudome M, Ito Y. Function of fusion regulatory proteins (FRPs) in immune cells and virus-infected cells. Rev Immunol. 2000;20:167–196. [PubMed] [Google Scholar]
- Wagner CA, Lang F, Broer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol. 2001;281:C1077–1093. doi: 10.1152/ajpcell.2001.281.4.C1077. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Genbacev O, Damsky CH, Fisher SJ. Oxygen regulates human cytotrophoblast differentiation and invasion: implications for endovascular invasion in normal pregnancy and in pre-eclampsia. J Repro Immunol. 1998;39:197–213. doi: 10.1016/s0165-0378(98)00022-9. [DOI] [PubMed] [Google Scholar]


