Abstract
Transforming growth factor (TGF)-β1 is a member of a superfamily of multifunctional cytokines involved in several pathological processes of the kidney, including fibrogenesis, apoptosis and epithelial-mesenchymal transition. These events lead to tubulointerstitial fibrosis and glomerulosclerosis. Less is known about TGF-β1-induced alterations of cell function. An important function of proximal tubular cells is reabsorption of filtered proteins, including albumin, via megalin-cubilin-dependent receptor-mediated endocytosis. In this study we used a well established cell culture model (proximal-tubule-derived opossum kidney (OK) cells) in order to test the hypothesis that TGF-β1 reduces megalin-cubilin-mediated endocytosis. Previously we have shown that albumin endocytosis in OK cells is mediated by megalin/cubulin. TGF-β1 led to a time- and dose-dependent downregulation of megalin-cubilin-mediated endocytosis without affecting two other transport systems tested. Binding, internalization and intracellular trafficking of the ligand albumin were affected. Decreased binding resulted from reduced cubilin and megalin expression in the 200 000 g membrane fraction. The underlying mechanism of TGF-β1 action does not involve mitogen-activated protein kinases, protein kinase C or A, or reactive oxygen species. In contrast, TGF-β1-induced downregulation of megalin-cubilin-mediated endocytosis was sensitive to inhibition of translation and transcription and was preceded by Smad2 and 3 phosphorylation. Dominant negative Smad2/3 constructs prevented the effect of TGF-β1. In conclusion our data indicate that enhanced levels of TGF-β1 occurring in various nephropathies can lead to downregulation of megalin-cubilin-dependent endocytosis. Probably, TGF-β1 leads to Smad2- and Smad3-dependent expression of negative regulators of receptor-mediated endocytosis.
Receptor-mediated endocytosis is an essential mechanism for the transport of a variety of macromolecules into cells as well as across epithelia (Mukherjee et al. 1997). Clathrin-mediated endocytosis is the predominant pathway for macromolecule uptake along epithelia (Mukherjee et al. 1997; Schmid, 1997; Marshansky et al. 1997; Christensen & Birn, 2002). An example of clathrin-mediated endocytosis is the uptake of filtered proteins by renal proximal tubular cells (Gekle et al. 1997; Gekle, 1998; Christensen & Birn, 2002). Renal proximal tubular protein reabsorption is of major importance because it prevents the loss of vitamins, hormones and amino acids (Christensen & Willnow, 1999), but at the same time it can induce tubulointerstitial inflammation and fibrosis (Burton & Harris, 1996; Jerums et al. 1997; Gekle, 1998). The mechanisms leading to protein-induced inflammation and fibrosis involve the expression of mediators such as RANTES, monocyte chemoattractant protein-1 (MCP-1), nuclear factor-κB (NF-κB) and mitogenic pathways (Burton & Harris, 1996; Dixon & Brunskill, 1999; Wang et al. 1999; Guijarro & Egido, 2001). One of the major receptors for proximal tubular protein endocytosis is the megalin- cubilin complex, which serves as a scavenger receptor (Christensen & Willnow, 1999) and includes reabsorption of albumin (Birn et al. 2000; Zhai et al. 2000; Verroust & Kozyraki, 2001), which binds to cubilin and megalin. Thus, the megalin-cubilin pathway can be monitored using albumin. The megalin-cubilin complex accepts a variety of ligands other than albumin (Christensen & Willnow, 1999; Nykjaer et al. 1999, 2001), including vitamin binding proteins, hormone binding proteins, hormones and light chains. In the case of vitamin D this pathway seems to be necessary for the final activation of the vitamin (Christensen & Willnow, 1999). Consequently, impairment of the megalin-cubilin pathway could lead to the urinary excretion of these proteins. Reduced megalin expression has been reported for Dent's disease, autosomal-dominant polycystic kidney disease and following exposure to aristocholic acid as reviewed by Verroust et al. (2002). The proximal-tubule-derived opossum kidney (OK) cell line has been shown to be a suitable model system for the study of megalin-cubilin-mediated, clathrin-dependent endocytosis of albumin (Dixon & Brunskill, 1999; Gekle et al. 1999; Zhai et al. 2000). As we have shown before, albumin endocytosis in OK cells can be inhibited by ligands for megalin (receptor-associated protein; RAP), cubilin (intrinsic factor) as well as by anti-megalin and anti-cubilin antibodies (Zhai et al. 2000). Thus, we used this cell line in order to determine the potential impact of TGF-β1 exposure on megalin-cubilin-mediated endocytosis.
TGF-β1 is a member of a superfamily of multifunctional cytokines involved in a wide array of biological activities, such as development and blood vessel modelling (Schiffer et al. 2000). In addition, TGF-β1 plays an important role in pathological processes, such as induction of extracellular matrix protein synthesis, compensatory renal growth, proximal tubular hypertrophy and epithelial- mesenchymal transdifferentiation (Kanda et al. 1993; Wolf et al. 1993; Border & Noble, 1994; Eickelberg et al. 1999). Epithelial- mesenchymal transdifferentiation can lead to a loss of typical epithelial features such as reabsorption or secretory transport and therefore not only support fibrosis but also decrease tubular function. Enhanced levels of TGF-β1 in pathophysiological conditions derive, for example, from upregulated synthesis in proximal tubular cells exposed to nephritogenic conditions (Van Kooten et al. 1999; Wolf et al. 2001) or from infiltrating macrophages. Various studies have shown that enhanced levels of TGF-β1 correlate positively with the amount of urinary protein excretion, i.e. proteinuria and albuminuria (Böttinger & Bitzer, 2002). Interestingly, a gene expression profiling approach revealed that TGF-β1 can affect the expression of several genes (either induction or repression) involved in endocytosis (Zavadil et al. 2001).
Proximal tubular cells are responsive to TGF-β1 (Wolf et al. 1993; Park et al. 2001). It has also been shown that proximal tubular cells produce TGF-β1 (Van Kooten et al. 1999; Wolf et al. 2001). Thus, the entire signalling cascade for TGF-β1 is present in the proximal tubule. Although altered TGF-β1 homeostasis is associated with renal diseases that lead to proteinuria and, furthermore, enhanced proximal tubular protein load can induce TGF-β1 formation in proximal tubular cells, it is not clear whether TGF-β1per se can affect proximal tubular protein endocytosis (Abbate & Remuzzi, 1999; Van Kooten et al. 1999). The aim of our present study was to determine whether exposure to TGF-β1 has an effect on megalin-cubilin-mediated protein uptake in a suitable cell culture system devoid of systemic factors.
METHODS
Materials
Minimal essential medium (MEM), Dulbecco's minimal essential medium (DMEM) and fetal calf serum were obtained from Biochrom, 12213 Berlin, Germany. All other chemicals were obtained from Sigma (Deisenhofen, Germany) if not stated otherwise. Human TGF-β1 was used in this study. Fluorescein isothiocyanate conjugated albumin (FITC-albumin; A-9771) was dialysed prior to the experiments in order to eliminate remaining free fluorescence and to ensure that the measured signal originated from albumin endocytosis. For the determination of Km and maximal binding (Bmax) of albumin binding, increasing concentrations of FITC-albumin were used. Ringer solution was composed of (mmol l−1): NaCl 122.5, KCl 5.4, CaCl2 1.2, MgCl2 0.8, Na2HPO4 0.8, NaH2PO4 0.2, glucose 5.5 and Hepes 10 (pH 7.4 at 37 °C).
Cell culture
Opossum kidney (OK) cells were kindly provided by Dr Biber (Deparment of Physiology, Zürich, Switzerland), and cultured (passage 83-100) as described before (Gekle et al. 1997). Porcine renal proximal tubule cells (LLC-PK1, passage 198-210, provided by Dr Gstraunthaler, Department of Physiology, Innsbruck, Austria) were grown in DMEM medium containing 10 % fetal calf serum and 24 mmol l−1 HCO3−. Cells were used 9 days after plating (confluent monolayers in 3.5 cm permeable supports with a pore diameter of 0.4 μm). Prior to incubation with TGFβ1, cells were washed once with serum-free medium and then cultivated in serum-free medium for 24 h. Serum-free medium indicates MEM in the case of OK cells or DMEM in the case of LLC-PK1-cells, both without any further additives. When not stated otherwise, TGF-β1 was added to the apical and basolateral compartments.
Transient transfection of the cells with dominant negative Smad2 (Smad2-S2A), dominant negative Smad3 (Smad3ΔSSVS) or the empty vector (pcDNA3) was performed with Effectene (Qiagen, Hilden, Germany) according to the manufacturer's instructions. A 1 μg quantity of DNA was used for each filter. Uptake experiments were performed 72 h after transfection. Equal transfection efficiencies were confirmed by parallel cotransfections using enhanced green fluorescent protein (EGFP). The constructs have been decribed in detail elsewhere (Souchelnytskyi et al. 1997; Choy et al. 2000).
Determination of collagen secretion
Collagen I and IV secretion was determined by enzyme-linked immunosorbent assay (ELISA). Media and collagen standards (Sigma) were incubated for 24 h in 96-well Nunc-Immuno Maxisorb plates (Nalge Nunc International, Naperville, IL, USA) followed by washing and blocking with 2 % bovine serum albumin. Subsequently the wells were incubated with rabbit antibody against collagen I or IV (1:1000; Biotrend, Cologne, Germany) for 1 h at room temperature. After three washes with 0.05 % Tween in phosphate-buffered saline (PBS), HRP-conjugated secondary antibody (1:5000; Biotrend, Cologne, Germany) was applied for 1 h at room temperature. After three further washes the wells were incubated with o-phenylenediamine (Sigma) and the reaction was stopped after 15 min with 1 N H2SO4. The absorbance at 490 nm was determined using a multiwell-multilabel reader. Cellular collagen was analysed by Western blot as described previously (Wohlfarth et al. 2003). Briefly, cells were lysed in ice-cold radioimmunopreciptation assay (RIPA) buffer and the lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Subsequently membranes were blotted with either rabbit anti-collagen type I or IV antibody (1:1000, Biotrend, Cologne, Germany). The primary antibody was detected using horseradish-peroxidase-conjugated secondary IgG (1:25 000) visualized by the Amersham Corp. ECL system.
Assessment of epithelial tightness
Epithelial tightness was determined by FITC-dextran equilibration essentially as described previously (Gekle et al. 1995b). Briefly, 1 g l−1 FITC-dextran (70 kD) was added to the basolateral compartment of permeable supports harbouring an OK-cell monolayer. The volume of the basolateral compartment was 2.5 ml and that of the apical compartment was 1.5 ml. Thus, complete equilibrium distribution is reached when 37.5 % of the dextran added basolaterally has moved to the apical compartment. After 12, 24, 48 and 72 h, aliquots of the apical and basolateral media were taken and the FITC-dextran concentration was determined using a using a multiwell-multilabel reader. The movement of FITC-dextran across the cell monolayer was compared with the movement across permeable supports without cells. As shown in Fig. 1 (left panel) FITC-dextran moves rapidly across permeable supports without cells and complete equilibration is almost reached after 72 h. In contrast, OK-cell monolayers prevented the movement of FITC-dextran almost completely (Fig. 1). Similar results, not shown here, were obtained with FITC-inulin. These data show that OK cells form a tight monolayer with respect to larger molecules. Addition of 3 μg l−1 TGFβ1 (Fig. 1) did not affect FITC-dextran movement during the time frame of this study. These data indicate that major epithelial-mesenchymal transdifferentiation with loss of epithelial tightness did not occur within the first 72 h. Measurements of transepithelial electrical resistance in order to assess monolayer tightness are not suitable because OK cells do not build up a high electrical resistance, similar to the proximal tubule in vivo which is a low-resistance epithelium.
Figure 1. TGF-β1 stimulates collagen secretion in OK cells (A) and OK cells form a tight monolayer (B).
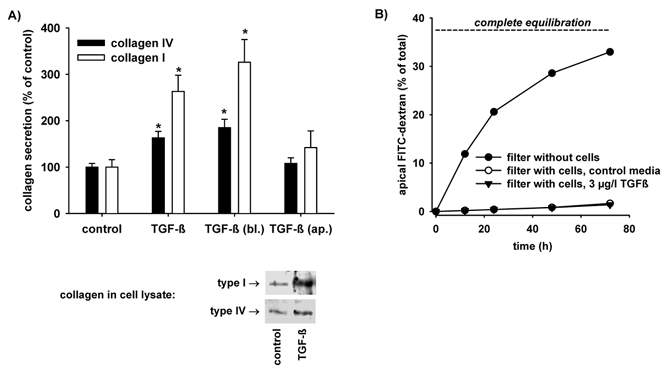
A, OK cells were incubated for 48 h with 3 μg l−1 TGF-β1. TGF-β1 stimulated the secretion of type I as well as type IV collagen and the basolateral (bl.) application of TGF-β1 was as effective as the application to both sides of the cell layer whereas apical (ap.) application exerted no significant effect. *P < 0.05 versus control; n = 6-9 for all data plotted. Western blot analysis of cell lysates indicates that TGF-β1 also enhanced collagen synthesis. B, diffusion of dextran across cell monolayers. Complete equilibrium distribution is reached when 37.5 % of the dextran added basolaterally has moved to the apical compartment. Fluorescein isothiocyanate conjugated (FITC)-dextran moves rapidly across permeable supports without cells and complete equilibration is almost reached after 72 h. In contrast, OK-cell monolayers prevented the movement of FITC-dextran almost completely. Addition of 3 μg l−1 TGFβ1 did not affect FITC-dextran movement during the time frame of this study. n = 3 for all values plotted.
Uptake and binding
Uptake experiments were performed as described earlier (Gekle et al. 1997). The monolayers were incubated with Ringer solution containing the indicated concentrations of FITC-bovine serum albumin (10 mg l−1 if not stated otherwise) at 37 °C for 15 min (for endocytosis) or at 4 °C for 15 min (= total binding to the cell surface; used for subtraction of material bound to the surface but not taken up by the cells when determining endocytosis) or 60 min (total binding at equilibrium). Less than 10 % of FITC-albumin uptake or binding is non-specific, i.e. cannot be prevented by an excess (10 g l−1) of non-labelled albumin. The amount of internalized substrate was determined by subtracting the portion of albumin bound to the cell surface at 4 °C from the total cell-associated albumin, determined at 37 °C. Unbound FITC-albumin was removed by rinsing eight times with ice-cold Ringer solution (Gekle et al. 1995a). Cells were lysed by detergent (Triton X-100, 0.1 % v/v in Mops solution, which guaranteed that all fluorescence measurements were performed at pH 7.4) and the cell-associated fluorescence was measured using a microplate spectrofluorometer (Victor2, Wallac, Turku, Finland). Protein was determined as described elsewhere (Lowry et al. 1951). The rate of fluid-phase endocytosis was determined by the uptake of FITC-dextran using the same protocol as for FITC-albumin uptake.
Albumin internalization
Cells were incubated with FITC-albumin for 60 min at 4 °C. After 60 min, equilibrium binding was achieved and >90 % of equilibrium binding was specific, i.e. could be prevented by an excess of non-labelled albumin. Monolayers were rinsed eight times with Ringer solution (4 °C) to remove unbound albumin and they were incubated for different time periods at 37 °C (for internalization of bound albumin). At the end of the internalization period the cells were incubated in acid stripping solution (50 mmol l−1 glycine + 2 mol l−1 urea, 30 g l−1 BSA, 100 mmol l−1 NaCl, pH 2.5) in order to remove FITC-albumin at the cell surface that had not been internalized. Strip-efficiency (i.e. percentage of cell-surface bound material removed by the strip procedure) was >96 %. Finally, cells were lysed and internalized FITC-albumin was determined. Internalized FITC-albumin is presented as a percentage of FITC-albumin bound to the cell surface during the initial 60 min of equilibrium binding.
Albumin degradation
In order to determine the degradation of FITC-albumin taken up, the amounts of trichloroacetic acid (TCA)-soluble and -insoluble fluorescence were determined after a 15 min uptake period (see above). The ratio of TCA-soluble/(TCA-insoluble + TCA-soluble) fluorescence represents a measure of degradation, which is independent of the actual uptake rate because the amount of degraded material (TCA-soluble) is always put in relation to the amount of material taken up (TCA-insoluble + TCA-soluble). A 1000 μl volume of TCA (10 %) was added to 1000 μl of the cell lysate or the Ringer solution for protein precipitation. After centrifugation at 9500 g for 10 min, 1000 μl of the supernatant was titrated to pH 7.4 with Mops buffer (1 mol l−1). The pellet was dissolved in 1 N NaOH and titrated to pH 7.4 using 1 M Mops buffer. In initial experiments we tested the fluorescence recovery after this treatment using fresh FITC-albumin. Fluorescence recovery was >90 %. TCA-soluble fluorescence in the incubation solutions was less than 1 % of total fluorescence.
Preparation of OK-cell membranes
OK cells were detached from the supports by scraping in imidazole buffer (0.3 M sucrose, 25 mM imidazole, 1 mM EDTA, pH 7.2) and centrifuged at 1000 g, 4 °C, for 5 min. The pellet was resuspended in 1.5 ml imidazole buffer, homogenized by five strokes at 1250 r.p.m. and centrifuged at 4000 g at 4 °C for 15 min. The pellet was homogenized and centrifuged again as described above. The supernatants were pooled and ultracentrifuged (L8-70M ultracentrifuge; Beckman Instruments, Fullerton, CA, USA) for 1 h, 4 °C, at 200 000 g. The final pellet was resuspended in sample buffer.
Western blotting of cubilin and megalin
SDS-PAGE gel electrophoresis was performed with 4-16 % polyacrylamide gradient gels and 3 % sodium dodecyl sulfate in the sample buffer. The proteins were electroblotted onto nitrocellulose membranes (Hyperbond ECL nitrocellulose; Amersham, Buckinghamshire, UK). After blotting, the membranes were blocked for 1 h in PBS containing 0.1 % Tween and 5 % low-fat dry milk, followed by washing three times for 25 min in PBS containing 0.1 % Tween. Subsequently, the blots were incubated with rabbit anti-rat cubilin antibodies or sheep anti-rat megalin antibodies diluted 1:2000 and 1:20 000, respectively, in PBS containing 0.1 %Tween, 1 % BSA and 2 mM sodium azide, overnight at 4 °C. The blots were washed as described above and incubated for 1 h with horseradish-peroxidase-conjugated secondary antibodies (goat anti-rabbit Ig 1:3000 or rabbit anti-sheep Ig 1:9000 were from DAKO A/S, Denmark). After washing, chemiluminescence substrate was added (ECL plus, Amersham) and quantified by the Fluor-S MultiImage system (BioRad). The signal intensity of the respective bands from controls and TGF-β1-treated samples was measured and compared for each single experiment. Thus, the signal intensity of the control bands in each experiment was normalized to 1 and the intensities of the TGF-β1-treated samples were expressed as percentages of the respective control. Previously we have shown (Zhai et al. 2000) that there is a virtual linear decrease of the signal as the amount of protein loaded onto the gel is reduced. As positive controls, affinity-purified cubilin and megalin were used. Polyclonal sheep anti-rat megalin and polyclonal rabbit anti-rat cubilin (L242 and L403) were obtained as previously described (Zhai et al. 2000).
Western blotting of phosphorylated Smad2 or Smad3
Equal amounts of cell lysates were loaded on a 10.5 % SDS-PAGE followed by transfer to nitrocellulose membrane. The membrane was blocked with 5 % non-fat dry milk for 1 h at room temperature and washed in TBS plus 0.5 % Tween-20. Incubation with the primary antibody (anti-phospho-Smad2, anti-phospho-Smad3, anti-Smad2 or anti-Smad3, 1:1000 each; Nakao et al. 1997; Ishisaki et al. 1999) was performed overnight at 4 °C, followed by incubation with the appropriate peroxidase-conjugated secondary antibody. Immunoreactive proteins were visualized by ECL. After incubation with anti-phospho-Smad2 antibody or anti-phospho-Smad3 antibody, the same blot was stripped and reprobed with anti-Smad2 or anti-Smad3 antibody, respectively.
Calculations and statistics
Curve fitting was performed according to the least-square-method using the SigmaPlot for Windows software (SPSS Inc., Chicago, IL, USA). Data are presented as mean values ± S.E.M.; n represents the number of Petri dishes. Cells of at least three passages were used for each experimental series. Significance of difference was tested by Student's unpaired t test or ANOVA as appropriate. Differences were considered significant if P < 0.05.
RESULTS
TGF-β1 enhances collagen secretion and reduces megalin-cubilin-mediated endocytosis of albumin in OK cells
OK cells have been shown to be a suitable model system to study megalin-cubilin-mediated endocytosis (Dixon & Brunskill, 1999; Gekle et al. 1999; Zhai et al. 2000). In order to test whether these cells respond to TGF-β1 we determined collagen secretion, which is typically enhanced by this cytokine (Eddy & Giachelli, 1995; Eickelberg et al. 1999; Wolf, 1999; Chin et al. 2001). As shown in Fig. 1A OK cells respond to 3 μg l−1 TGF-β1 with enhanced collagen type I and type IV secretion. Furthermore, basolateral addition of TGF-β1 exerted a similar effect when compared with the addition of TGF-β1 to the basolateral as well as to the apical side, indicating that TGF-β1 acts via the basolateral membrane in OK cells. This polarized action of TGF-β1 is in agreement with the described basolateral expression of TGF-β1 receptors in proximal tubules (Wang et al. 2000) as well as with the basolateral action of TGF-β1 in cultured proximal tubular cells (Tian & Phillips, 2002). Figure 1B shows that OK cells form a tight monolayer with the capacity to separate the basolateral from the apical compartment. Western blot analyses of cell lysates (Fig. 1A, bottom) indicates that TGF-β1 also stimulated collagen synthesis. The effects on collagen I were more pronounced as compared with collagen IV. The underlying molecular mechanisms are not clear at the moment. Since TGF-β1 is a profibrotic cytokine, the preferential increase of collagen I (located in the interstitial space) as compared with collagen IV (located in the basement membrane) is not surprising.
As shown in Fig. 2A exposure to TGF-β1 for 48 h induced a concentration-dependent decrease of endocytosis. A similar effect was observed in another proximal-tubule-derived cell line, LLC-PK1, indicating that the observed effect is not restricted to OK cells. The reduction of endocytosis was also observed when TGF-β1 was added to the basolateral side only (Fig. 2A). In order to exclude a general downregulation of apical transport mechanisms, we determined the effect of TGF-β1 on apical fluid-phase endocytosis, using FITC-dextran and secondary active apical amino acid uptake using cycloleucine. As shown in Fig. 2B, 3 μg l−1 TGF-β1 did not affect these transport processes, thereby excluding a general downregulation of apical transport. The time course of action of TGF-β1 on albumin endocytosis is shown in Fig. 3. A 2 h period of exposure exerted no significant effect, whereas after 5 h, endocytosis was reduced significantly to 80 % of control. Thereafter endocytosis decreased with increasing time of exposure.
Figure 2. TGF-β1 reduces albumin endocytosis.
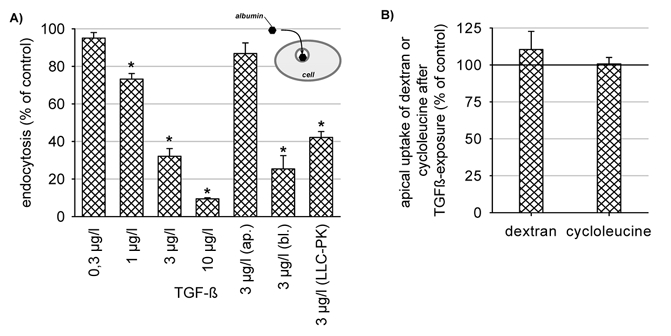
A, concentration-dependent downregulation of albumin endocytosis. Incubation time = 48 h. Basolateral (bl.) application of TGF-β1 was as effective as the application to both sides of the cell layer. Apical (ap.) addition had virtually no effect. TGF-β1 induced a similar effect in another proximal tubule-derived cell line (LLC-PK1). B, secondary active cycloleucine uptake and dextran uptake were not affected. *P < 0.05 versus the respective control; n = 6-15 for all data plotted.
Figure 3. Time course of the TGF-β1 action on albumin endocytosis.
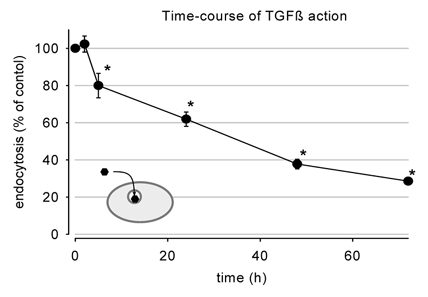
TGF-β1 (3 μg l−1) was effective after 5 h incubation. *P < 0.05 versus control; n = 6-15 for all data plotted.
TGF-β1 affects binding and internalization of albumin
In order to learn more about the potential mechanisms of TGF-β1-induced reduction of endocytosis we determined albumin binding to the apical membrane of OK cells. After 48 h exposure to 3 μg l−1 TGF-β1 binding was reduced, as shown in Fig. 4. Kinetic analysis revealed that the overall apparent binding affinity as well as maximum binding capacity was reduced. Also in OK cells, it was shown recently that albumin interacts with megalin and cubilin (Birn et al. 2000; Zhai et al. 2000). Binding to cubilin shows high affinity whereas binding to megalin shows lower affinity (Birn et al. 2000). These data do not exclude the existence of further low-affinity binding sites, which, under physiological conditions, play only a minor role in endocytosis. Thus, it is conceivable that the observed decrease in binding affinity results from a disproportionate reduction in cubilin/megalin expression and putative further low-affinity binding site expression. Western blot analysis of cubilin and megalin expression (Fig. 4B) revealed that cubilin and megalin expression was reduced. Thus, the effect of TGF-β1 can be explained, at least in part, by altered binding. However, this does not exclude mechanisms such as internalization or intracellular trafficking. The effect of TGF-β1 on internalization is shown in Fig. 5A. After 48 h exposure to 3 μg l−1 TGF-β1, the internalization of prebound albumin, expressed as a percentage of bound albumin, was reduced significantly. These data indicate that trafficking of bound ligands is also affected by TGF-β1. In order to strengthen this hypothesis we determined intracellular degradation of albumin as a percentage of the albumin taken up. This measure gives some indirect information about the delivery of albumin to degradation sites, such as lysosomes, and therefore indirect information about trafficking. As shown in Fig. 5B, 48 h exposure to 3 μg l−1 TGF-β1 led to a dramatic reduction of albumin degradation, indicating that intracellular trafficking is indeed affected by TGF-β1.
Figure 4. Effect of TGF-β1 on albumin binding.
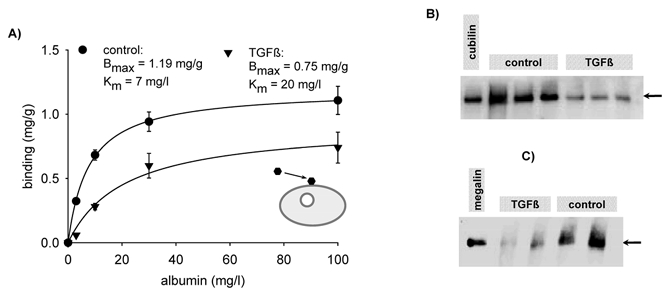
A, 48 h exposure to 3 μg l−1 TGF-β1 reduced albumin binding to the apical surface (only FITC-albumin binding that can be displaced by excess of non-labelled albumin is plotted). Kinetic analysis shows a decrease of the apparent affinity as well as of the maximum binding capacity. n = 6-15 for all data plotted. B, 48 h exposure to 3 μg l−1 TGF-β1 led to a decrease of cubilin (arrow) expression in OK cells. The plot shows cubilin expression from three control filters and three TGF-β1-treated filters. This experiment was repeated three times with virtually identical results. C, 48 h exposure to 3 μg l−1 TGF-β1 led to a decrease of megalin (arrow) expression in OK cells. The blot shows megalin expression from two control filters and two TGF-β1-treated filters. This experiment was also repeated three times with similar results. The blots were quantified as described in Methods.
Figure 5. TGF-β1 reduces the rate of internalization of albumin bound to the apical membrane of OK cells (A) and exposure to TGF-β1 for 48 h reduces the rate of degradation of albumin taken up by OK cells (B).
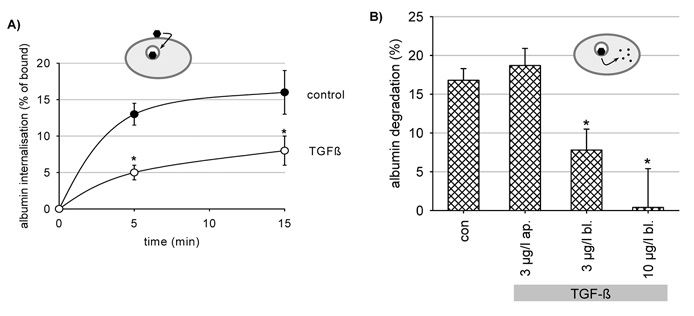
A, cells were exposed to 3 μg l−1 TGF-β1 for 48 h. *P < 0.05 versus control; n = 6 for all data plotted. B, percentage albumin degradation represents the fraction of albumin degraded as compared to the amount of albumin taken up and is therefore independent of the uptake rate. *P < 0.05 versus control; n = 9 for all data plotted. Ap., exclusive apical addition of TGF-β1; Bl., exclusive basolateral addition of TGF-β1.
Which pathways mediate the effect of TGF-β1 on endocytosis?
The classical way of action of TGF-β1 is via Smad proteins, which are transcriptional modulators (Schiffer et al. 2000). However, during recent years it has become evident that TGF-β1 may also use other pathways, such as mitogen-activated protein kinases, protein kinase C (PKC) and reactive oxygen species (Choi, 2000; Inoki et al. 2000; Chin et al. 2001; Ha et al. 2001). We applied inhibitors of the extracellular-signal-regulated-kinase pathway (PD98059, U0126), the p38-kinase pathway (SB203580), the PKC pathway (BIM), the PKA pathway (H89) and the PI-3-kinase pathway (Ly294002). As shown in Fig. 6A none of these inhibitors prevented the action of TGF-β1. Thus, it seems unlikely that any of these pathways are involved. Next we tested scavengers of reactive oxygen species (dimethylthiourea, N-acetyl-cysteine, Tiron). Again, none of these inhibitors prevented the action of TGF-β1. Surprisingly, Tiron reduced endocytosis by itself (Fig. 6B). The underlying mechanism is not clear at the moment.
Figure 6. The effect of TGF-β1 (3 μg l−1 for 48 h) on albumin endocytosis is not affected by inhibitors of protein kinases or phosphatidylinositol (PI)-3-kinase (A) and the effect of TGF-β1 (3 μg l−1 for 48 h) on albumin endocytosis is not affected by scavengers of reactive oxygen species (B).
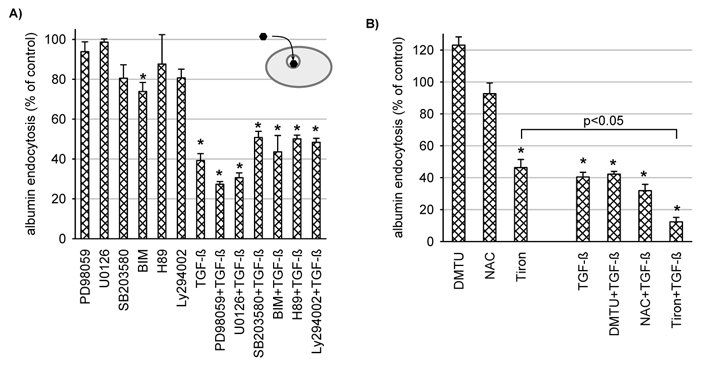
A, the inhibitors were present for 48 h. PD98059 (25 μmol l−1) and U0126 (10 μmol l−1) are inhibitors of extracellular-signal-regulated-kinases; SB203580 (10 μmol l−1) is an inhibitor of the p38-kinase; BIM (bisindolylmaleiimide, 100 nmol l−1) is an inhibitor of protein kinase C; H89 (1 μmol l−1) is an inhibitor of protein kinase A; Ly294002 (10 μmol l−1) is an inhibitor of PI-3-kinase. *P < 0.05 versus control; n = 6-15 for all data plotted. B, the scavengers were present for 48 h; 10 mmol l−1 Tiron, 1 mmol l−1 NAC (N-acetyl-cysteine), 250 μmol l−1 DMTU (dimethylthiourea). *P < 0.05 versus control; n = 6 for all values plotted.
Finally, we tested whether the effect of TGF-β1 depends on protein synthesis. We used cycloheximide to block translation, and actinomycin D to block transcription. As shown in Fig. 7, TGF-β1 did not reduce endocytosis in the presence of these two substances. Actinomycin D reduced endocytosis by itself rather dramatically and therefore the data are not easy to interpret. However, in the presence of Tiron, which reduced endocytosis even further (Fig. 6B), TGF-β1 was still effective. These data indicate that protein synthesis is necessary for the effect of TGF-β1. Since TGF-β1 affects protein synthesis via the Smad pathway (Schiffer et al. 2000) it is conceivable that TGF-β1 leads to an activation of this pathway in OK cells.
Figure 7. The effect of TGF-β1 (3 μg l−1 for 48 h) on albumin endocytosis depends on protein synthesis.
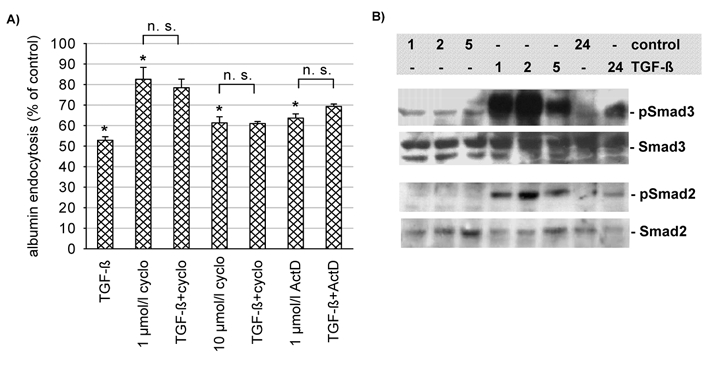
A, when translation (cyclo, cycloheximide) or transcription (Act D, actinomycin D) were inhibited no significant effect of TGF-β1 could be observed. *P < 0.05 versus control; n = 9 for all values plotted. B, TGF-β1 (3 μg l−1) led to a time-dependent increase of Smad2 and Smad3 phosphorylation with virtually no change in protein expression. The maximum effect of TGF-β1 was observed after 2 h, but phosphorylation was still increased after 24 h as compared to control. This experiment has been performed twice with similar results.
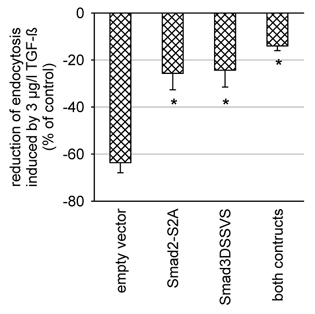
As shown in Fig. 7B, TGF-β1 (3 μg l−1) induced Smad2 and Smad3 phosphorylation within 1 h (see lanes 4-6). TGF-β1-induced phosphorylation of both Smad2 and Smad3 started to decline after 2 h. However, even after 24 h there still was an enhanced level of phosphorylated Smad2 and Smad3 in TGF-β1-treated cells as compared with controls (lanes 7 and 8). The time course of Smad phosphorylation precedes the decrease in albumin endocytosis, which is in agreement with the working model of a Smad-induced downregulation of albumin endocytosis. Furthermore, transient transfection of the cells with dominant-negative Smad2 (Smad2-S2A; Souchelnytskyi et al. 1997) and/or Smad3 (Smad3ΔSSVS; Choy et al. 2000; Fig. 8) reduced the effect of TGF-β1 on endocytosis significantly.
Figure 8. Transient transfection of the cells with dominant negative Smad2 (Smad2-S2A, 1 μg per filter), dominant negative Smad3 (Smad3ΔSSVS) or both reduced the effect of TGF-β1 (3 μg l−1, 48 h) significantly as compared to transfection with the empty vector. Transfection was performed 72 h prior to the uptake experiments. *P < 0.05 versus empty vector; n = 6 for all values plotted.
DISCUSSION
We characterized the effects of TGF-β1 on endocytic events in a well-established cell culture model for proximal tubular cells, the OK cells, which express the important megalin-cubilin-mediated endocytic pathway as shown previously (Zhai et al. 2000). We first determined whether these cells do react in a profibrotic way, i.e. with enhanced collagen synthesis, in the presence of TGF-β1. Our data show that this is indeed the case. Basolateral TGF-β1 enhanced the secretion of collagen type I and type IV, and at the same time led to a decrease in receptor-mediated endocytosis. Thus, TGF-β1 acts at the basolateral membrane. Possibly, TGF-β1-induced alterations of the expression of genes relevant for endocytosis contribute to the observed effects (Zavadil et al. 2001). Because the heterodimer megalin-cubilin is a multiligand receptor (Christensen & Birn, 2002) pathophysiological alterations of megalin- cubilin-mediated endocytosis can affect the homeostasis of a variety of important compounds. The clearance of peptide hormones, such as parathyroid hormone, epidermal growth factor or insulin, from tubular fluid would be slowed down leading to enhanced intratubular steady-state concentrations as suggested for the CLC-5 knockout mouse (Piwon et al. 2000). Finally, the cytoplasmic tail of megalin has been suggested to contain a signalling function and therefore altered megalin handling might lead to inappropriate signalling (Christensen & Birn, 2002).
The data presented here show that enhanced levels of TGF-β1 lead to reduced megalin-cubilin-mediated endocytosis at concentrations well within the range expected to occur in vivo, underscoring the potential pathophysiological significance of our results. Furthermore, TGF-β1, applied in the time frame of this study, does not induce a general downregulation of cell function, because secondary active cycloleucine uptake and dextran endocytosis were not affected. Kinetic analysis of equilibrium binding indicates that the overall apparent binding affinity as well as maximum binding capacity for albumin are decreased by TGF-β1. Reduced binding affinity can be explained by the more pronounced downregulation of ‘high-affinity’ binding sites such as cubilin and megalin as compared to other binding sites with lower affinity which, under physiological conditions, play only a minor role.
Additionally, TGF-β1 led to a reduction in the internalization rate of prebound ligand. Because internalization depends on megalin, which in contrast to cubilin has a transmembrane domain, the reduced internalization rate shows that not only cubilin-mediated endocytosis, but also megalin-mediated endocytosis is affected. This is further supported by the action of TGF-β1 on degradation of internalized albumin. TGF-β1 reduced the rate of degradation of internalized albumin, indicative of altered postendocytic trafficking. The precise nature of these alterations is not known at present. However, it is unlikely that TGF-β1 led to a generalized defect in vesicle trafficking because fluid-phase endocytosis, determined by dextran uptake, was not affected. Possibly, TGF-β1 altered the receptor-ligand sorting machinery leading to a slower transition of internalized ligand to the sites of degradation.
The time course of action of TGF-β1, as well as the dependence on protein synthesis, suggest that ‘rapid’ signalling cascades, for example a direct action of protein kinases or reactive oxygen species, are not involved. This notion is further supported by our pharmacological data. Thus, it is most likely that the classical Smad pathway (Schiffer et al. 2000) mediates the effects of TGF-β1. Indeed we were able to show that TGF-β1 induces phosphorylation of Smad2 and Smad3 in our cells. We tested the hypothesis of Smad2/3-mediated inhibition of endocytosis using dominant negative Smad2 and 3. Indeed, transient transfection with dominant negative Smad2 and 3 reduced the action of TGF-β1 significantly. We also tried to characterize the TGF-β receptors expressed in OK cells. Unfortunately, we were not able to obtain consistent results (data not shown), a problem arising most probably from antibody specificity or the level of receptor expression. However, the prompt effects of TGF-β1 on Smad phosphorylation indicate that TGF-β1 acts via the classical type I and type II receptors.
In conclusion our data show that enhanced levels of TGF-β1, occurring with various forms of nephropathies, can lead to downregulation of the important megalin-cubilin-dependent receptor-mediated endocytosis. Possibly, TGF-β1 leads to the Smad2/3-dependent expression of negative regulators of receptor-mediated endocytosis. Of course, the effects of TGF-β1 may not be restricted to renal proximal tubular cells and endocytosis in other tissues may also be reduced in situations of elevated TGF-β1.
Acknowledgments
The excellent technical assistance of Pia K. Nielsen and Yvonne Kehl is fully appreciated. The work was supported in part by the Deutsche Forschungsgemeinschaft (Ge 905/3-4), by the Wilhelm-Sander-Stiftung (grant to M.G.), the University of Würzburg, the Danish Medical Research Council, the University of Aarhus and the Novo Nordisk Foundation. We thank Dr C. H. Heldin (Uppsala) und Dr P. t. Dijke (Amsterdam) for providing anti-Smad antibody.
REFERENCES
- Abbate M, Remuzzi G. Proteinuria as a mediator of tubulointerstitial injury. Kidney Blood Press Res. 1999;22:37–46. doi: 10.1159/000025907. [DOI] [PubMed] [Google Scholar]
- Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, Willnow T, Moestrup SK, Christensen EI. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest. 2000;105:1353–1361. doi: 10.1172/JCI8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- B öttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- Burton C, Harris KpG. The role of proteinuria in the progression of chronic renal failure. Am J Kidney Dis. 1996;27:765–775. doi: 10.1016/s0272-6386(96)90512-0. [DOI] [PubMed] [Google Scholar]
- Chin BY, Mohsenin A, Li SX, Choi AMK, Choi ME. Stimulation of pro-alpha 1 (I) collagen by TGF-beta 1 in mesangial cells: role of the p38 MAPK pathway. Am J Physiol Renal Physiol. 2001;280:F495–504. doi: 10.1152/ajprenal.2001.280.3.F495. [DOI] [PubMed] [Google Scholar]
- Choi ME. Mechanism of transforming growth factor-beta1 signaling: Role of the mitogen-activated protein kinase. Kidney Int. 2000;58:S53–S58. [PubMed] [Google Scholar]
- Choy L, Skillington J, Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol. 2000;149:667–682. doi: 10.1083/jcb.149.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen EI, Birn H. Megalin and cubilin: Multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Willnow TE. Essential role of megalin in renal proximal tubule for vitamin homeostasis. J Am Soc Nephrol. 1999;10:2224–2236. doi: 10.1681/ASN.V10102224. [DOI] [PubMed] [Google Scholar]
- Dixon R, Brunskill NJ. Activation of mitogen pathways by albumin in kidney proximal tubule epithelial cells: Implications for proteinuric states. J Am Soc Nephrol. 1999;10:1487–1497. doi: 10.1681/ASN.V1071487. [DOI] [PubMed] [Google Scholar]
- Eddy AA, Giachelli CM. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int. 1995;47:1546–1557. doi: 10.1038/ki.1995.218. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Köhler E, Reichenberger F, Bertschin S, Woodtli T, Erne P, Perruchoud AP, Roth M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-β1 and TGF-β3. Am J Physiol. 1999;276:L814–824. doi: 10.1152/ajplung.1999.276.5.L814. [DOI] [PubMed] [Google Scholar]
- Gekle M. Renal proximal tubular albumin reabsorption: The daily prevention of albuminuria. News Physiol Sci. 1998;13:5–11. doi: 10.1152/physiologyonline.1998.13.1.5. [DOI] [PubMed] [Google Scholar]
- Gekle M, Drumm K, Mildenberger S, Freudinger R, Gaßner B, Silbernagl S. Inhibition of Na+-H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. J Physiol. 1999;520:709–721. doi: 10.1111/j.1469-7793.1999.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Schwerdt G, Silbernagl S. Albumin endocytosis in OK cells: Dependence on actin and microtubules, regulation by protein kinases. Am J Physiol. 1997;272:F668–677. doi: 10.1152/ajprenal.1997.272.5.F668. [DOI] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Silbernagl S. Endosomal alkalinization reduces Jmax and Km of albumin receptor-mediated endocytosis in OK cells. Am J Physiol. 1995a;268:F899–906. doi: 10.1152/ajprenal.1995.268.5.F899. [DOI] [PubMed] [Google Scholar]
- Gekle M, Pollock CA, Silbernagl S. Time- and concentration-dependent biphasic effect of ochratoxin A on growth of proximal tubular cells in primary culture. J Pharmacol Exp Ther. 1995b;275:397–404. [PubMed] [Google Scholar]
- Guijarro C, Egido J. Transcription factor-kB (NF-kB) and renal disease. Kidney Int. 2001;59:415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- Ha H, Yu MR, Lee HB. High glucose-induced PKC activation mediates TGF-β1 and fibronectin synthesis by peritoneal mesothelial cells. Kidney Int. 2001;59:463–470. doi: 10.1046/j.1523-1755.2001.059002463.x. [DOI] [PubMed] [Google Scholar]
- Inoki K, Haneda M, Ishida T, Mori H, Maeda S, Koya D, Sugimoto T, Kikkawa R. Role of mitogen-activated protein kinases as downstream effectors of transforming growth factor-b in mesangial cells. Kidney Int. 2000;58:S76–S80. doi: 10.1046/j.1523-1755.2000.07712.x. [DOI] [PubMed] [Google Scholar]
- Ishisaki A, Yamato K, Hashimoto S, Nakao A, Tamaki K, Nonaka K, Ten Dijke P, Sugino H, Nishihara T. Differential inhibition of Smad6 and Smad7 on bone morphogenetic protein- and activin-mediated growth arrest and apoptosis in B cells. J Biol Chem. 1999;274:13637–13642. doi: 10.1074/jbc.274.19.13637. [DOI] [PubMed] [Google Scholar]
- Jerums G, Panagiotopoulos S, Tsalamandris C, Allen TJ, Gilbert RE, Comper WD. Why is proteinuria such an important risk factor for progression in clinical trials. Kidney Int. 1997;52:S87–S92. [PubMed] [Google Scholar]
- Kanda S, Igawa T, Taide M, Eguchi J, Nakamura M, Nomata K, Yamada J, Kanetake H, Saito Y. Transforming growth factor-beta in rat kidney during compensatory renal growth. Growth Regul. 1993;3:146–150. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marshansky V, Bourgoin S, Londono I, Bendayan M, Maranda B, Vinay P. Receptor-mediated endocytosis in kidney proximal tubules: Recent advances and hypothesis. Electrophoresis. 1997;18:2661–2676. doi: 10.1002/elps.1150181423. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, Ten Dijke P. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, De La Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proc Natl Acad Sci U S A. 2001;98:13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Choi HJ, Lee JH, Woo CH, Kim JH, Han HJ. High glucose inhibits renal proximal tubule cell proliferation and involves PKC, oxidative stress, and TGF-beta 1. Kidney Int. 2001;59:1695–1705. doi: 10.1046/j.1523-1755.2001.0590051695.x. [DOI] [PubMed] [Google Scholar]
- Piwon A, Günther W, Schwake M, Bösl MR, Jentsch TJ. CIC-5 Cl−-channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- Schiffer M, Von Gersdorff G, Bitzer M, Susztak K, Böttinger EP. Smad proteins and transforming growth factor-β signaling. Kidney Int. 2000;58:S45–S52. doi: 10.1046/j.1523-1755.2000.07708.x. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: An integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Souchelnytskyi S, Tamaki K, Engstrom U, Wernstedt C, Ten Dijke P, Heldin CH. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-beta signaling. J Biol Chem. 1997;272:28107–28115. doi: 10.1074/jbc.272.44.28107. [DOI] [PubMed] [Google Scholar]
- Tian YC, Phillips AO. Interaction between the transforming growth factor-beta type II receptor/Smad pathway and beta-catenin during transforming growth factor-beta1-mediated adherens junction disassembly. Am J Pathol. 2002;160:1619–1628. doi: 10.1016/s0002-9440(10)61109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kooten C, Langers AMJ, Bruijn JA, Daha MR. Role of tubular cells in progressive renal disease. Kidney Blood Press Res. 1999;22:53–61. doi: 10.1159/000025909. [DOI] [PubMed] [Google Scholar]
- Verroust PJ, Birn H, Nielsen R, Kozyraki R, Christensen EI. The tandem endocytic receptors megalin and cubilin are important proteins in renal pathology. Kidney Int. 2002;62:745–756. doi: 10.1046/j.1523-1755.2002.00501.x. [DOI] [PubMed] [Google Scholar]
- Verroust PJ, Kozyraki R. The roles of cubilin and megalin, two multiligand receptors, in proximal tubule function: possible implication in the progression of renal disease. Curr Opin Nephrol Hypertens. 2001;10:33–38. doi: 10.1097/00041552-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Wang SN, Lapage J, Hirschberg R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int. 2000;57:1002–1014. doi: 10.1046/j.1523-1755.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rangan GK, Tay YC, Harris DCH. Induction of monocyte chemoattractant protein-1 by albumin is mediated by nuclear factor kB in proximal tubule cells. J Am Soc Nephrol. 1999;10:1204–1213. doi: 10.1681/ASN.V1061204. [DOI] [PubMed] [Google Scholar]
- Wohlfarth V, Drumm K, Mildenberger S, Freudinger R, Gekle M. Protein uptake disturbs collagen homeostasis in proximal tubule-derived cells. Kidney Int Suppl. 2003;84:103–109. doi: 10.1046/j.1523-1755.63.s84.13.x. [DOI] [PubMed] [Google Scholar]
- Wolf G. Vasoactive factors and tubulointerstitial injury. Kidney Blood Press Res. 1999;22:62–70. doi: 10.1159/000025910. [DOI] [PubMed] [Google Scholar]
- Wolf G, Hannken T, Schroeder R, Zahner G, Ziyadeh FN, Stahl RA. Antioxidant treatment induces transcription and expression of transforming growth factor beta in cultured renal proximal tubular cells. FEBS Lett. 2001;488:154–159. doi: 10.1016/s0014-5793(00)02403-0. [DOI] [PubMed] [Google Scholar]
- Wolf G, Müller E, Stahl RAK, Ziyadeh FN. Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J Clin Invest. 1993;92:1366–1373. doi: 10.1172/JCI116710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai XY, Nielsen H, Birn H, Drumm K, Mildenberger S, Freudinger R, Moestrup SK, Verroust P, Christensen EI, Gekle M. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of Opossum kidney. Kidney Int. 2000;58:1523–1533. doi: 10.1046/j.1523-1755.2000.00314.x. [DOI] [PubMed] [Google Scholar]


