Abstract
Conflicting reports have appeared concerning the cell cycle regulation of telomerase activity and its possible repression during quiescence and cell differentiation. We have reexamined these issues in an attempt to uncover the basis for the discrepancies. Variations in extracted telomerase activity during the cell cycle are not observed in cells sorted on the basis of DNA content. Variations are observed in cells synchronized using some biochemical cell cycle inhibitors, but only with those agents where cellular toxicity is evident. A progressive decline in telomerase activity is observed in cells whose growth rate is reduced from seven to eight population doublings per week to one to two doublings per week. Telomerase is largely absent in cells that truly exit the cell cycle and do not divide over the 7-day period. Although it is not necessary for all cell types to regulate telomerase in the same way, we conclude that in the immortal cultured cell lines examined, extracted telomerase activity does not change significantly during progression through the stages of the cell cycle. Telomerase activity generally correlates with growth rate and is repressed in cells that exit the cell cycle and become quiescent.
Somatic cells passaged in culture reach the end of their replicative capacity after a limited number of population doublings (1). The process of telomere shortening has been proposed as a regulatory mechanism that controls the replicative capacity of eukaryotic cells (2–5). Telomeres shorten with each cell division due to incomplete replication at the end of the chromosome (6, 7). In the absence of a mechanism to compensate for the end-replication problem, the process of telomere shortening is repeated with successive cell divisions, providing progeny cells with progressively shortened telomeres until the time when cells become senescent and stop dividing (2–5).
For cells to overcome senescence or M1 (mortality stage 1) (8), the actions of p53 and pRb-like proteins must be blocked. Such cells continue to proliferate until M2 (mortality stage 2), when telomere lengths are thought to become critically shortened (8–11). A rare immortal cell occasionally arises from this population of cells in crisis (M2), and this proliferation-competent, immortal cell usually expresses telomerase activity (12–14). Although many human tissues lack detectable telomerase activity, there is now a large number of examples of normal diploid cells (lymphocytes and a variety of epithelial cells) that can express telomerase activity while proliferating in vivo (15–23). However, the observation that telomeres from these tissues still shorten as a function of donor age suggests that the functional activity of the telomerase in these cells may be sufficient to slow but not prevent telomere erosion. Approximately 85% of all primary human cancers have telomerase activity (13, 24, 25). The identification of those clinical situations in which the detection of telomerase activity has diagnostic or prognostic utility and the development of in situ techniques to distinguish telomerase contribution by normal vs. cancer cells represents areas being actively investigated in many laboratories.
We have reported that telomerase-competent cells down-regulate telomerase activity when they become quiescent, and the process is reversible upon the initiation of proliferation and reentry into the cell cycle (26). Telomerase activity is repressed during the process of differentiation in a variety of telomerase-positive cultured cell types (26–29). Because many lineages exit the cell cycle when they differentiate, the repression of telomerase activity could either be a specific component of the differentiation program or simply a consequence of the same mechanism that down-regulates telomerase in quiescent cells.
Analysis of telomerase-positive cells sorted by flow cytometry without drug treatment showed approximately equivalent amounts of telomerase activity at each stage of the cell cycle (26). However, in cells synchronized using chemical compounds, Zhu et al. (30) suggested that telomerase activity increases in S phase and shows a sharp decrease during mitosis. In addition, they reported that quiescent and dividing cells have similar levels of telomerase activity.
We have resolved this discrepancy by treating cells with a panel of chemical compounds to determine if the observed changes in telomerase activity at different stages of the cell cycle were drug-induced. In the present study, we found that telomerase activity remained constant in cells treated with any of five G1/S blockers and did not increase as cells progressed through S phase. Cells arrested in mitosis with colcemid did not show a decrease in telomerase activity. However, both nocodazole and doxorubicin—agents that produced mitotic arrest with decreased telomerase activity—also showed toxic effects. In addition, we found that telomerase activity varied with growth rate and was repressed in quiescent cells. Using methods that reduced proliferation >85% from one population doubling per day to one doubling per week, telomerase activity decreased as the rate of proliferation declined. Conditions that caused cells to become quiescent and not divide at all produced a substantial decrease in telomerase activity. Finally, we determined that telomerase activity has a half-life of >24 hr in almost all cell lines tested. Taken together, these results show that extracted telomerase activity does not vary with the cell cycle in dividing cells, directly correlates with growth rate, and is down-regulated as cells exit the cell cycle.
MATERIALS AND METHODS
Human Cell Lines and Culture.
Human mammary epithelial cells [HME 32(273)-1], immortalized by mutant p53 (R-273-H), were maintained in serum-free medium as described (31). The human cell types used in the present study were DU145 (prostate carcinoma), H1299 (non-small cell lung carcinoma), RCC23 (renal cell carcinoma), SW480 (colon adenocarcinoma), HT1080 (fibrosarcoma), and simian virus 40 T antigen immortalized IMR90 diploid lung fibroblast derivatives IDH4 and SW39I. These cells were grown in a 4:1 mixture of DMEM and medium 199, containing 10% iron-supplemented calf serum (HyClone) and 25 μg/ml gentamicin (Sigma). IDH4 cells, immortalized using a dexamethasone-inducible promoter upstream of the simian virus 40 T antigen, were cultured in the same medium supplemented with 1 μM dexamethasone, unless otherwise indicated (8). HT1080 and DU145 cells were incubated in 2-fold dilutions of serum (10%, 5%, 2.5%. 1.25%, 0.6%, 0.3%, and 0%) in medium with gentamicin for 7 days.
Drug Treatments.
HT1080 and SW480 cells were treated with a variety of cell cycle inhibitors to arrest cells at specific stages of the cell cycle. Many agents were used under the conditions described by Zhu et al. (30). Cells were exposed to G1/S phase blockers [5 mM hydroxyurea/10 μM 5-fluorouracil/10 μM methotrexate/1 μM cytosine β-d-arabinofuranoside (Ara-C)/combination of thymidine and aphidicolin (5 mM and 5 μg/ml, respectively)] for 16–20 hr. After the G1/S blockade, cells were released at varying times to permit progression through the cell cycle. Cells were arrested in G2/M following treatment for 16–20 hr with colcemid (50 μg/ml), doxorubicin (10 μM), or nocodazole (40 ng/ml). Methotrexate, Ara-C, aphidicolin, doxorubicin, and nocodazole were solubilized in dimethyl sulfoxide and subsequently diluted into medium with a final dimethyl sulfoxide concentration of <0.01% (26). All other compounds were prepared in water. Cells were either harvested directly to assay for telomerase activity (unsorted) or separated by flow cytometry into stages of the cell cycle based on DNA content (sorted). Some cell lines were treated with 300 μg/ml cycloheximide (Sigma) to block protein synthesis and harvested for the determination of telomerase activity at various time points. All telomerase assays were repeated one or two times.
Telomerase Assays.
Detection of telomerase activity using the telomeric repeat amplification protocol (TRAP) in cultured cells involves the addition of TTAGGG repeats by telomerase to an oligonucleotide (TS), and the subsequent PCR amplification of these extension products with both the forward (TS) and reverse (CX) primers (13, 32). The TRAP-eze telomerase detection kit was used as recommended by the manufacturer (Oncor) with minor modifications (33). Briefly, cell pellets were stored at −80°C until lysis was performed. The lysis buffer contained 1% Nonidet P-40 and 0.25 mM sodium deoxycholate to increase the efficiency of extraction (34). Cells were lysed, left on ice for 30 min, and centrifuged at 14,000 × g for 20 min at 4°C. The supernatant was flash-frozen and stored at −80°C. For the PCR reaction, 2 μl of extract (corresponding to 100-1000 cells) was combined with the 48-μl reaction mixture supplied with the kit and 2 units of Taq DNA polymerase (GIBCO/BRL). After incubation at room temperature for 30 min for the telomerase extension reaction, samples were heated to 92°C for 3 min to inactivate telomerase followed by PCR amplification as described (33). PCR products were electrophoresed on 10% polyacrylamide gels, and the gels analyzed and quantitated using the PhosphorImaging system and imagequant software from Molecular Dynamics.
Flow Cytometry.
HT1080 and SW480 cells were rinsed with PBS containing 1% serum to remove residual growth medium (26). Rinsed cells were resuspended at a concentration of 1 × 106 cells per ml in PBS containing 1% serum and 1.8 μg/ml Hoechst 33342 and incubated at 37°C for 30–45 min. Cells were maintained on ice until sorted into G1, S, and G2/M phases of the cell cycle based on DNA content and cell viability on a fluorescence-activated cell sorter (FACScan, Becton Dickinson). Sorted cells were placed on ice for a maximum of 10–15 min, pelleted, and either frozen or processed for the TRAP assay as described (26, 32, 33).
RESULTS
Detection of Telomerase Activity Throughout the Cell Cycle.
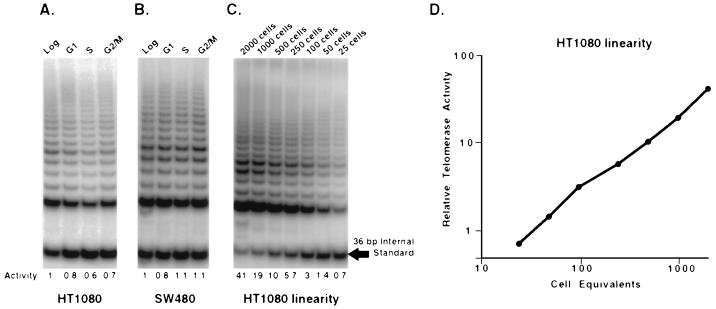
The immortal, pseudodiploid fibrosarcoma cell line HT1080 exhibits only slight variation in the levels of the telomerase activity at different stages of the cell cycle (G1, S, G2/M) when sorted by FACS (26). However, it has been reported that there is a cell cycle regulation of telomerase activity based on chemically synchronized SW480 cells (30). To directly compare these apparently conflicting results, we FACS sorted SW480 cells at different stages of the cell cycle for telomerase activity determination. Fig. 1 shows that there appears to be no significant differences in telomerase activity at any stage of the cell cycle (G1, S, G2/M) in FACS-sorted HT1080 or SW480 cells. Fig. 1 also shows a representative 2-fold dilution series of a cellular extract of HT1080, which demonstrates the utility of the internal standard. Taq polymerase becomes limiting as the number of substrate molecules increases (plateau). The amount of the 36-bp internal standard has been adjusted so that plateau is not reached after 27 PCR cycles in samples containing low levels of telomerase. However, plateau does occur as higher levels of telomerase produce more elongation products. Competition between these elongation products and the internal standard for amplification of Taq polymerase then results in reduced amounts of the internal standard at plateau. The ratio of the intensity of the signal from the telomerase ladder divided by the intensity of the signal from the internal standard provides an effective means to quantitate these effects. Fig. 1D demonstrates that this ratio is linear over the range of telomerase activities used in these experiments.
Figure 1.
Telomerase activity is detected in all stages of the cell cycle in HT1080 and SW480 cells. HT1080 (A) and SW480 (B) cells were sorted by flow cytometry based on DNA content and cell viability into the different stages of the cell cycle. Sorted cells were pelleted and lysed, and aliquots containing the equivalent of 100 cells were analyzed for telomerase activity using the TRAP-eze telomerase detection kit. Telomerase activity produces the 6-bp ladder of amplification products. The ratio of the telomerase ladder to the 36-bp internal standard permits relative quantitation of each lane. Below each lane is the relative telomerase activity ratios as compared with the unsorted fraction. (C) A 2-fold dilution series of an HT1080 extract is shown. Note that above 250 cells, increasing telomerase extension products can be detected as an increasingly effective competitor for the amplification of the 36-bp internal standard. (D) The data in C is plotted as the ratio of telomerase products to internal standard (relative telomerase activity) vs. cell equivalents to demonstrate the linearity of the assay.
Telomerase Activity After Treatment with Cell Cycle Arresting Agents.
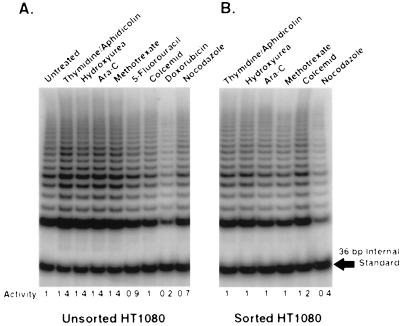
There have been reports that telomerase activity is increased in S phase cells and is down-regulated in cells in the G2/M phase of the cell cycle (30). In the present study, we duplicated and extended these experiments using a variety of drugs to synchronize cells (Fig. 2). None of the drugs had any direct effect on telomerase activity when added to cellular extracts (data not shown). We also used flow cytometry to sort, and thus analyze separately, synchronized cells at the different stages of the cell cycle in addition to sampling the entire population of semisynchronized cells as in the previous experiments (30). Fig. 2 shows the results of TRAP assays on unsorted (Fig. 3A) and sorted (Fig. 3B) HT1080 cells after treatment with various drugs. There is no significant difference in the levels of telomerase activity between cells treated with G1/S inhibitors (thymidine/aphidicolin, hydroxyurea, Ara-C, methotrexate, or 5-fluorouracil) in unsorted cells or populations sorted for G1 DNA content (Fig. 2 A and B). However, cells treated with G2/M blockers showed variable results. Telomerase activity was reduced 5-fold following doxorubicin treatment. Although there was no inhibition in unsorted or sorted colcemid-treated cells, nocodazole-treated cells showed a slightly reduced telomerase activity compared with the unsorted cells (Fig. 2).
Figure 2.
The effects of cell cycle blocking agents on telomerase activity. HT1080 cells were treated with various compounds designed to inhibit cell cycle progression. Cells were either harvested directly for use in the TRAP assay (unsorted) (A) or processed for flow cytometry (sorted) into G1 (thymidine/aphidicolin, hydroxyurea, Ara-C, methotrexate), or G2/M (colcemid, nocodazole) fractions (B). FACS sorting of the arrested cells was performed in an attempt to increase the synchrony of the experiment. Cells treated with doxorubicin were not sorted as they appeared dead by flow cytometry analysis. Below each lane is the relative telomerase activity ratios as compared with the untreated fraction in A.
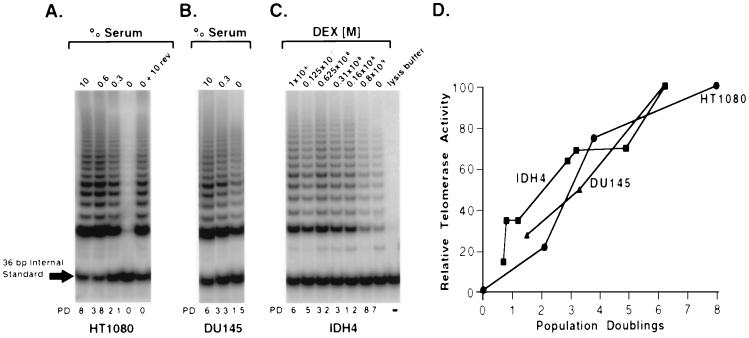
Figure 3.
Telomerase activity correlates with the rate of cell proliferation in immortal human cells. (A and B) Cells were rinsed and resuspended in medium without serum to obtain serum-free conditions and then plated in medium containing different concentrations of serum. Subconfluent cell cultures were harvested at 7 days, counted, and analyzed for telomerase activity. Serum-containing medium (10%) was added to a duplicate culture 0% serum plate for 2–3 days to test for reversibility of the quiescent block (0% + 10% rev). A gel from a representative experiment is shown, and the average number of cell divisions (population doubling) from several experiments is indicated below each lane. (A) HT1080 cells appear to undergo reversible quiescence in 0% serum. Telomerase activity appears proportional to growth rate. (B) DU145 cells are resistant to quiescence induced by serum-deprivation. The decrease in telomerase activity with decreased cell division may represent the combined effects of decreased growth rate and toxicity. (C) IDH4 cells were rinsed with medium without dexamethasone and plated in medium containing dexamethasone in a series of 2-fold dilutions. IDH4 cells also show a decrease in telomerase activity with the rate of proliferation. (D) Quantitation of telomerase activity and the rate of cell division are plotted showing a relatively linear relationship for each cell line tested. Data from several independent experiments have been combined.
Doxorubicin, a drug widely used as a chemotherapeutic agent for treating cancer, has been shown to increase the rate of mutation of the p53 gene while increasing the proportion of cells in S and G2/M (35). After incubation of cells in 9 μM doxorubicin for only 60 min, accumulation of cells in S and G2/M stages was reported to occur in cells that were still viable (only 10%) 24 hr after release from the drug (35). Zhu et al. (30) treated SW480 cells with 10 μM doxorubicin over a 72-hr period and observed a sharp decrease in telomerase activity. We treated both HT1080 and SW480 cells with 10 μM doxorubicin and observed large amounts of cell death after 12–16 hr. Only adherent cells were harvested for telomerase analysis, and no sorted cells could be obtained as most cells appeared dead by both FACS analysis and incorporation of trypan blue (data not shown). Thus, the reduction of telomerase activity in the unsorted samples (Fig. 2A) is probably due to a significant portion of the harvested population being nonviable or dead, as reported (36).
Unsorted nocodazole treated HT1080 cells showed no significant differences in the level of telomerase activity when compared with untreated, asynchronous HT1080 cells (Fig. 2A). Sorted nocodazole-arrested cells had a 2.5-fold decrease in telomerase activity (Fig. 2B) but also had 2-fold less protein per cell than the asynchronous HT1080 population (data not shown). Both before and after sorting, nocodazole treated cells exhibited a 4–5 fold decrease in cloning efficiency versus untreated HT1080 cells and a 2–3-fold decrease when compared with colcemid treated cells (data not shown). These results suggest that the decrease in telomerase activity seen in the sorted cells probably results from decreased cell viability. There was no decrease in protein levels or telomerase activity from cells treated with colcemid in either sorted or unsorted populations. The reduction in viability found in nocodazole-treated cells is further indicated by the results following release. Nocodazole treated cells released and then examined for telomerase activity after 6 and 12 hr showed a gradual increase in both overall protein concentration per cell and telomerase activity (data not shown). These results indicate a likely drug-induced toxicity when cells are treated with nocodazole that is not detected in cells treated with colcemid. Thus, the only G2/M inhibitors that produce decreased telomerase activity were those that also had toxic effects.
The change in telomerase activity during S phase was examined in cells released from the G1/S blockade and assayed at various time points. The G1/S phase blocks for thymidine/aphidicolin and for hydroxyurea were reversible with >95% cell viability (data not shown). No variation was observed in the levels of telomerase activity as thymidine/aphidicolin or hydroxyurea treated HT1080 cells progressed through each phase of the cell cycle after release from the blockade (data not shown). SW480 cells synchronized at G1/S with thymidine/aphidicolin or hydroxyurea also showed no change in telomerase activity during S phase after release from the block (data not shown).
The Effects of Quiescence and the Rate of Cell Proliferation on Telomerase Activity.
There have been reports that the levels of telomerase activity in quiescent cells are similar to actively dividing cells (30). Because we have shown (26) that telomerase activity in several cell culture model systems decreases when cells are subjected to reversible quiescence, we compared telomerase activity and cell proliferation rates in cells growing in 10% serum to those growing in reduced or no serum to explore the relationship between proliferative status and telomerase activity.
In the absence of serum, the HT1080 cells became quiescent and telomerase activity was dramatically reduced (Fig. 3A). Whereas HT1080 cells in 10% serum divided eight times per week, the cells in 0.3% serum only divided twice, and the level of telomerase activity was proportional to the rate of cell division (Fig. 3 A and D). Relative telomerase activity was quantitated as the ratio of the internal standard to the telomerase products and expressed as a percentage of the untreated or 0 day samples (Fig. 3D). DU145 cells did not exhibit a quiescent state and continued to divide in the absence of serum, although at a slower rate than the cells in 10% serum containing medium (Fig. 3B). Similar to HT1080 cells, the level of telomerase activity in DU145 cells decreased as the rate of proliferation declined over a 7-day period (Fig. 3D). However, DU145 cells incubated in 0% and 0.3% serum showed an increase in cell death/apoptosis and a decrease in cloning efficiency (data not shown). Because the decrease in telomerase activity might be partially due to this toxicity, we were unable to rigorously determine if telomerase activity varies with growth rate in DU145 cells. IDH4 cells are an in vitro immortalized cell line that requires dexamethasone for the expression of simian virus 40 T antigen and maintenance of the immortal phenotype (8). When IDH4 cells were incubated for 10 days in decreasing concentrations of dexamethasone, both the rate of proliferation and telomerase activity decreased (Fig. 3 C and D). Thus, telomerase-positive cells need only be in the proliferative pool to express telomerase activity and the rate of cell proliferation and the level of telomerase activity are directly related.
Half-Life of Telomerase Activity in Cultured Cells.
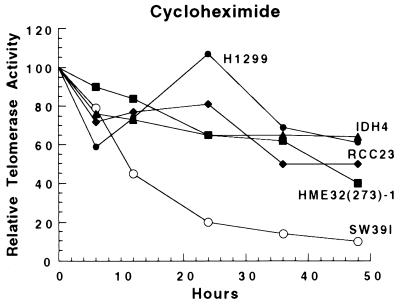
Recently, we showed that telomerase activity in HT1080 and HL60 (a human promyelocytic leukemia cell line) cells was stable and had a half-life of over 24 hr (26). To determine if this was a general result, five additional cell lines from a variety of lineages were tested. Cells were analyzed for telomerase activity in the presence of cycloheximide, a potent inhibitor of protein synthesis. Although protein synthesis was inhibited by >90% after 6 hr (data not shown), telomerase activity was decreased with a half-life of >24 hr for all of the telomerase-positive cell lines with the exception of SW39I (Fig. 4). In several different experiments, the telomerase activity in SW39I cells appeared to be biphasic, with a sharp initial decline during the first 12 hr followed by a more typical decline during the next 36 hr. There are many potential explanations for the more rapid disappearance of telomerase in SW39I cells. The rapid disappearance of telomerase activity in differentiating HL-60 promyelocytic leukemia cells suggests that some pathways for the proteolytic targeting of telomerase may exist (26). SW39I cells may have such a pathway that is activated by cycloheximide, or cycloheximide may simply be more toxic to SW39I than the other cells and produce a decrease in activity similar to that seen in nocodozole or doxorubicin-treated cells. Regardless of the mechanism for the decreased half-life in SW39I cells, it is clear that the half-life of telomerase activity in six of seven cell lines tested was >24 hr (26), indicating that telomerase is a highly stable protein/RNA complex.
Figure 4.
Telomerase appears to be a highly stable protein complex in a variety of cell types. Cultured cells were treated with the protein synthesis inhibitor, cycloheximide, for the indicated times and harvested for detection of telomerase activity. Data from two or three separate experiments with each cell line have been combined.
DISCUSSION
There are contradictory results in the literature about the cell cycle regulation of telomerase activity. Some studies have suggested that telomerase activity is regulated at each stage of the cell cycle (30), yet we and others have found that telomerase activity does not vary significantly at the different stages of the cell cycle (26, 37). We have also found a decrease in telomerase activity in quiescent cells (26). However, Zhu et al. (30) suggested that quiescent cells have the same level of telomerase activity as actively dividing cells.
Here, we have shown that extracted telomerase activity in FACS sorted cells is detected at approximately equal amounts at each stage of the cell cycle. Zhu et al. (30) found an increase (2–3-fold) in telomerase activity during S phase in cells treated with thymidine/aphidicolin or hydroxyurea and a substantial decrease in G2/M phase cells when treated with nocodazole or doxorubicin. We found no increase in telomerase activity in G1/S arrested cells when treated with thymidine/aphidicolin, hydroxyurea, Ara-C, or methotrexate. We also observed no significant change in the level of telomerase activity as cells progress through S phase or G2/M. Some of the chemical compounds used by Zhu et al. (30) to arrest cells at different stages of the cell cycle have toxic effects on cells that decrease the levels of telomerase activity. Cells treated with nocodazole or doxorubicin showed toxic effects, resulting in a decrease in telomerase activity and protein concentration, as also recently reported by Faraoni et al. (36). Colcemid, which also blocks cells in mitosis, was not toxic under the conditions used and did not decrease telomerase activity or protein concentration. These data would, at least in part, explain this conflict. In addition, an internal standard is needed to obtain reliable estimates of the relative levels of telomerase activity. We believe that 2-fold changes in the quantitation of telomerase activity using the PCR-based TRAP assay should be interpreted with caution. Also, because telomerase activity appears to be highly stable, with a half-life of ≈24 hr in a variety of cell lines, it seems unlikely that during an 18–24 hr complete cell cycle, the level of telomerase activity would be significantly decreased as cells enter mitosis. Therefore, we conclude that in cell extracts telomerase activity does not vary during different stages of the cell cycle. However, it is possible that in intact cells, telomerase action on telomeres is restricted to specific times during the cell cycle.
Under conditions of serum deprivation and/or T antigen removal, HT1080 and IDH4 cells become quiescent and down-regulate telomerase activity. In both cases, the rate of proliferation and the level of telomerase activity appear to show a direct relationship. In contrast to the work presented here and previously (26), Zhu et al. (30) reported that serum-deprived DU145 cells have the same level of telomerase activity as normal cells. There are several possible explanations for these apparently contradictory results. Some cell types may be resistant to quiescence induced by serum-deprivation. In our hands, DU145 cells continue to divide in low or no serum with a small increase in cell number accompanied by an increase in cell death/apoptosis. Zhu et al. (30) report that there is no change in the number of cells under conditions of serum deprivation. However, the figure shown by Zhu et al. (30) clearly shows proliferating clusters of cells in the absence of serum. We suggest that their cells were not quiescent and the apparent lack of increased cell numbers was a consequence of apoptosis balancing cell division. Thus, the conditions and cell lines used by Zhu et al. (30) to induce quiescence may have been insufficient to address the issue of quiescence and telomerase activity.
Although it is unlikely that telomerase is necessary in nonproliferating cells as telomeres do not continue to shorten in the absence of cell division, quiescent and differentiated cells may have alternative means of regulating the expression and activity of telomerase to compensate for the lack of cellular proliferation (38–40). Quiescent cells may down-regulate the expression of telomerase by repressing transcription or assembly, as the kinetics of the loss of activity are not significantly different from the half-life studies (26). However, the kinetics of the down-regulation of telomerase activity in differentiating cells suggests that additional mechanisms may be involved (26–29). The half-life data suggests that telomerase is a highly stable molecule, yet differentiating cells appear to down-regulate telomerase within the first 24 hr of being stimulated to differentiate. The regulation and/or repression of telomerase in differentiating cells may thus include mechanisms such as the direct physical interaction of telomerase with regulatory proteins or degradation of the RNA or protein components. Understanding the regulation of telomerase using cell culture systems may provide important insights for developing novel cancer diagnostic and therapeutic approaches.
Acknowledgments
We acknowledge Paul H. Pompa and Patricia McChesney for excellent technical assistance, and Drs. Lauren S. Gollahon, James C. Norton, and Harold Werbin for helpful discussions. This work was supported by research grants from the National Institutes of Health (AG01228), the Geron Corporation (Menlo Park, CA), and the National Institute for Aging [postdoctoral support for S.E.H. (AG05747)].
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: TRAP, telomeric repeat amplification protocol; M1 and M2, mortality stages 1 and 2; Ara-C, cytosine β-d-arabinofuranoside; FACS, fluorescence-activated cell sorter.
References
- 1.Hayflick L. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 2.de Lange T, Shiue L, Myers R M, Cox D R, Naylor S L, Killery A M, Varmus H E. Mol Cell Biol. 1990;10:518–526. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greider C W. BioEssays. 1990;12:363–369. doi: 10.1002/bies.950120803. [DOI] [PubMed] [Google Scholar]
- 4.Harley C B, Fletcher A B, Greider C W. Nature (London) 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 5.Harley C B. Mutat Res. 1991;256:271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 6.Watson J D. Nature (London) 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 7.Olovnikov A M. J Theor Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 8.Wright W E, Pereira-Smith O M, Shay J W. Mol Cell Biol. 1989;9:3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allsopp R C, Vaziri H, Patterson C, Goldstein S, Younglai E V, Futcher A B, Greider C W, Harley C B. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy M Z, Allsopp R C, Futcher A B, Greider C W, Harley C B. J Mol Biol. 1992;225:951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- 11.Shay J W, Pereira-Smith O M, Wright W E. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- 12.Counter C M, Avillon A A, LeFeuvre C E, Stewart N G, Greider C W, Harley C B, Bacchetti S. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 14.Shay J W, Wright W E, Brasiskyte D, Van Der Haegen B A. Oncogene. 1993;8:1407–1413. [PubMed] [Google Scholar]
- 15.Broccolli D, Young J W, de Lange T. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Counter C M, Gupta J, Harley C B, Leber B, Bacchetti S. Blood. 1995;85:2315–2320. [PubMed] [Google Scholar]
- 17.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek M A, Shay J W, Ishoika S, Yamakido M. J Immunol. 1995;155:3711–3715. [PubMed] [Google Scholar]
- 18.Hiyama E, Hiyama K, Tatsumoto N, Kodama T, Shay J W, Yokoyama T. Int J Oncol. 1996;9:453–458. doi: 10.3892/ijo.9.3.453. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao R, Sharma H W, Ramakrishnan S, Keith E, Narayanan R. Anticancer Res. 1997;17:827–832. [PubMed] [Google Scholar]
- 20.Klingelhutz A J, Foster S A, McDougall J K. Nature (London) 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 21.Kyo S, Takaura M, Kohama T, Inoue M. Cancer Res. 1997;57:610–614. [PubMed] [Google Scholar]
- 22.Ramirez R D, Wright W E, Shay J W, Taylor R S. J Invest Dermatol. 1997;108:113–117. doi: 10.1111/1523-1747.ep12285654. [DOI] [PubMed] [Google Scholar]
- 23.Tsao J-I, Zhao Y, Lukas J, Yang X, Shah A, Press M, Shibata D. Clin Cancer Res. 1997;3:627–631. [PubMed] [Google Scholar]
- 24.Shay J W, Wright W E. Curr Opin Oncol. 1996;8:66–71. doi: 10.1097/00001622-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Shay J W, Bacchetti S. Eur J Cancer. 1997;5:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 26.Holt S E, Wright W E, Shay J W. Mol Cell Biol. 1996;16:2932–2939. doi: 10.1128/mcb.16.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albannell J, Han W, Mellado B, Gunawardane R, Scher H I, Dmitrovosky E, Moore M A S. Cancer Res. 1996;56:1503–1508. [PubMed] [Google Scholar]
- 28.Bestilny L J, Brown C B, Miura Y, Robertson L D, Riabowol K T. Cancer Res. 1996;56:3796–3802. [PubMed] [Google Scholar]
- 29.Sharma H W, Sokoloski J S, Perez J R, Maltese J Y, Sartorelli A C, Stein C A, Nichols G, Khaled Z, Telang N T, Narayanan R. Proc Natl Acad Sci USA. 1995;92:12343–12346. doi: 10.1073/pnas.92.26.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X, Kumar R, Mandal M, Sharma N, Sharma H W, Dhingra U, Sokoloski J A, Hsiao R, Narayanan R. Proc Natl Acad Sci USA. 1996;93:6091–6095. doi: 10.1073/pnas.93.12.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gollahon L S, Shay J W. Oncogene. 1996;12:715–725. [PubMed] [Google Scholar]
- 32.Piatyszek M A, Kim N W, Weinrich S L, Keiko H, Hiyama E, Wright W E, Shay J W. Methods Cell Sci. 1995;17:1–15. [Google Scholar]
- 33.Holt S E, Norton J C, Wright W E, Shay J W. Methods Cell Sci. 1996;18:237–248. [Google Scholar]
- 34.Norton J C, Gollahon L S, Holt S E, Wright W E, Shay J W. Proc Am Assoc Can Res. 1997;38:504. (abstr.). [Google Scholar]
- 35.Kwok T T, Mok C H, Menton-Brennan L. Cancer Res. 1994;54:2834–2836. [PubMed] [Google Scholar]
- 36.Faraoni I, Turriziani M, Masci G, De Vecchis L, Shay J W, Bonmassar E, Graziani G. Clin Cancer Res. 1997;3:579–585. [PubMed] [Google Scholar]
- 37.Mantell L L, Greider C W. EMBO J. 1994;13:3211–3217. doi: 10.1002/j.1460-2075.1994.tb06620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright W E, Piatyszek M A, Rainey W E, Byrd W, Shay J W. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Bryan T M, Engelzou A, Gupta J, Bachetti S, Reddel R R. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohmura H, Tahara H, Suzuki M, Ide T, Shimizu M, Yoshida M A, Tahara E, Shay J W, Barrett J C, Oshimura M. Jpn J Cancer Res. 1995;86:899–904. doi: 10.1111/j.1349-7006.1995.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]