Abstract
We have identified silver tetrafluoroborate (AgBF4) as an excellent promoter for the activation of various glycosyl donors including glycosyl halides, trichloroacetimidates, thioimidates, etc. Easy handling and no requirement for azeotropic dehydration prior to application makes AgBF4 especially beneficial in comparison to the commonly used AgOTf. Selective activation of glycosyl halides or thioimidates over thioglycosides or n-pentenyl glycosides, including simple sequential one-pot syntheses, has been also demonstrated. Versatility of glycosyl thioimidates was further explored by converting these intermediates into a variety of other classes of glycosyl donors.
With increasing demand for the synthesis of biologically important and therapeutically active oligosaccharides and glycoconjugates, efforts to expand the arsenal of glycosylation methods and techniques have emerged. In spite of significant recent advances in the areas of stereoselective glycosylation1,2 and expeditious oligosaccharide synthesis,3,4 the construction of complex oligosaccharides with high efficiency and complete stereoselectivity remains a difficult task.
As a part of a program to develop new methods and strategies for glycochemistry, we became interested in glycosyl thioimidates, a class of glycosyl donors with the generic leaving group SCR1=NR2.5,6 We have already reported the synthesis of S-benzoxazolyl (SBox)7,8 and S-thiazolinyl (STaz)9,10 glycosides and evaluated their properties in stereoselective glycosylations and expeditious oligosaccharide syntheses. Metal salt-based promoters were shown to provide efficient activation of the thioimidoyl moiety for glycosylation.8,11 Amongst a variety of metal salts investigated, arguably, silver trifluoromethanesulfonate (AgOTf) was one of the best choices for the activation of the SBox and STaz moieties for a variety of synthetic applications.
Beyond the scope of our own research program, AgOTf has been commonly employed as an activator for many other classes of glycosyl donors including glycosyl bromides,12–14 chlorides,15 trichloroacetimidates,16 and seleno glycosides.17 In spite of wide applicability and high versatility of AgOTf, there are some significant drawbacks that limit the usage of this promoter in synthesis. In a majority of applications, AgOTf requires fresh activation by repetitive co-evaporation with toluene followed by extended drying under vacuum directly prior to use. In addition, we noticed that the quality of commercial reagent may significantly vary depending on the supplier and the batch. It should be noted that the use of AgOTf as an acidic additive in NIS-promoted glycosidation of thioglycosides does not require preactivation of AgOTf.
Bearing in mind these drawbacks, we wondered if any other silver salts could be used to achieve an efficient glycosidation of a variety of glycosyl donors and would produce reproducible results without the requirement of the preactivation or azeotropic dehydration. Herein we present a report that demonstrates the use of silver tetrafluoroborate (AgBF4) as a potent promoter for the activation of various types of glycosyl donors. It should be noted that AgBF4 has already been used in glycosylations, yet these applications, including alcoholysis of glycosyl bromides,18,19 synthesis of C-glycosides from chlorides,20 glycosidation of 2-deoxy thioglycosides,21 and α-stereoselective glycosidation of 1,2-anhydro sugars,22 were scarce and far apart. In this context, the combination of Cp2ZrCl2-AgBF4 in benzene has proven to be a powerful promoter combination for the activation of tetra-O-benzyl-D-mannosyl fluoride.23
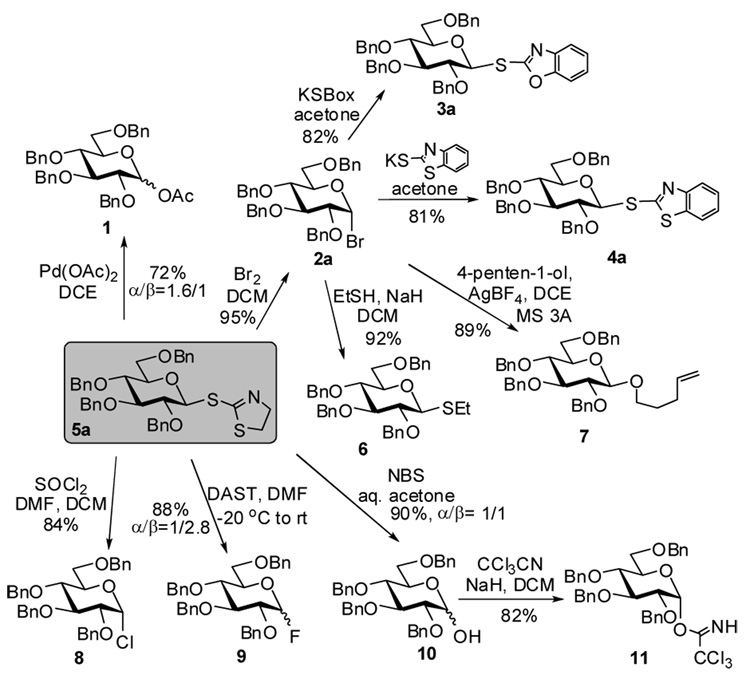
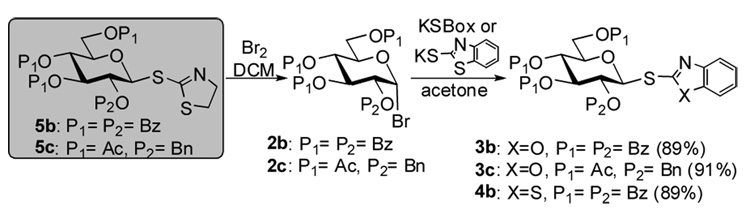
In order to generate a broad array of glycosyl donors we investigated whether thiazolinyl 2,3,4,6-tetra-O-benzyl-1-thio-β-D-glucopyranoside 5a10 could serve as a common precursor. These studies were successful and we discovered that the STaz glycoside 5a can be directly converted into the following glycosyl donors: acetate 1, bromide 2a, chloride 8, fluoride 9, and hemiacetal 10 in excellent yields (84–95%, Scheme 1). Moreover, glycosyl bromide 2a was then used as a precursor for the next synthetic steps to obtain benzoxazolyl, benzthazolyl, and ethyl thioglycosides (3a, 4a, and 6), as well as n-pentenyl glycoside 7 – all with exclusive β stereoselectivity. The hemiacetal derivative 10 was converted into the corresponding glycosyl trichloroacetimidate 11 as shown in Scheme 1. Synthesis of a range of differently protected glycosyl thioimidates has been similarly accessed and is depicted in Scheme 2. Yields given are over-all yields for two synthetic steps.
Scheme 1.
Conversion of a common STaz building block (5a) into a range of glycosyl donors
Scheme 2.
Conversion of the STaz glycosides 5b,c into thioimidates 3b,c and 4.
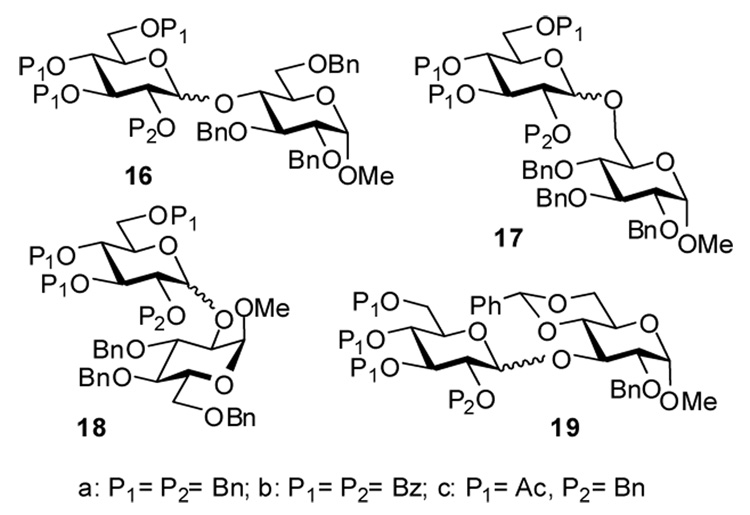
Having synthesized a library of glycosyl donors, we performed a number of test glycosylation reactions using AgBF4 as a promoter (Table 1). Since we wanted to determine whether the glycosylations could be performed without preactivation of AgBF4, all reactions were performed with commercial reagent as received, without further conditioning. Glycosidation of glycosyl acetate 1 with glycosyl acceptor 13 in the presence of AgBF4 did not proceed (entry 1). A similar coupling in the presence of BF3-Et2O, a conventional promoter for the activation of glycosyl acetates,24 gave the disaccharide 17a (Figure 1) in 91% yield, although it required 16 h for the reaction to complete. We determined that the reaction time of this reaction could be significantly reduced (16 h vs. 5 h, entries 2 and 3) by adding 20–50 mol % of AgBF4. In these couplings, even higher yield of 17a (95%) and an improved stereoselectivity was detected.
Table 1.
AgBF4 promoted glycosylations of glycosyl acceptors 12–15.
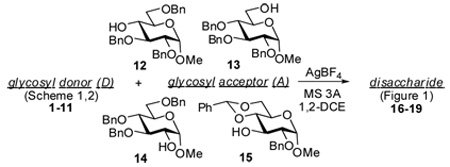 | ||||||
|---|---|---|---|---|---|---|
| entry | D | A | time | product | yield, % | α/β ratio |
| 1 | 1 | 13 | 24 h | 17a | -a | - |
| 2b | 1 | 13 | 16 h | 17a | 91 | 1.4/1 |
| 3c | 1 | 13 | 5 h | 17a | 95 | 2.0/1 |
| 4 | 2a | 13 | 5 min | 17a | 92 | 2.4/1 |
| 5 | 2b | 13 | 5 min | 17b | 96 | β only |
| 6 | 2b | 14 | 24 h | 18b | 82 | β only |
| 7 | 3a | 13 | 10 min | 17a | 89 | 1.2/1 |
| 8 | 3b | 13 | 10 min | 17b | 91 | β only |
| 9 | 3b | 14 | 15 min | 18b | 84 | β only |
| 10 | 3c | 13 | 15 min | 17c | 91 | 1.6/1 |
| 11 | 4a | 15 | 5 min | 19a | 87 | 7.4/1 |
| 12 | 4b | 13 | 20 min | 17b | 90 | β only |
| 13 | 5a | 13 | 5 min | 17a | 95 | 1.3/1 |
| 14 | 5a | 14 | 5 min | 18a | 92 | 1.4/1 |
| 15 | 5b | 13 | 10 min | 17b | 90 | β only |
| 16 | 5c | 13 | 20 min | 17c | 75 | 1.2/1 |
| 17 | 6 | 13 | 24 h | 17a | -a | - |
| 18d | 6 | 13 | 24 h | 17a | 91 | 1.2/1 |
| 19e | 6 | 13 | 10 min | 17a | 92 | 1.2/1 |
| 20 | 7 | 13 | 24 h | 17a | -a | - |
| 21d | 7 | 13 | 24 h | 17a | 89 | 1.0/1 |
| 22e | 7 | 13 | 20 min | 17a | 76 | 1.2/1 |
| 23 | 8 | 13 | 5 min | 17a | 97 | 1.6/1 |
| 24 | 8 | 12 | 10 min | 16a | 91 | 1/3.4 |
| 25 | 9 | 13 | 20 h | 17a | 81 | 2.0/1 |
| 26 | 9 | 15 | 1.5 h | 19a | 81 | 2.3/1 |
| 27 | 10 | 13 | 24 h | 17a | -a | - |
| 28 | 11 | 13 | 5 min | 17a | 80 | 1/1.5 |
| 29 | 11 | 12 | 2 days | 16a | 65 | 1/5.4 |
no product formation was detected; all glycosylations were promoted with AgBF4 except the following:
3.0 equiv. of BF3-Et2O was used
3.0 equiv. of BF3-Et2O and 0.5 equiv. of AgBF4 was used
2.0 equiv. of NIS was used
2.0 equiv. of NIS and 0.5 equiv. of AgBF4 was used.
Figure 1.
Structures of Disaccharides 16–19
Glycosidation of glycosyl bromides 2a and 2b could be efficiently accomplished with AgBF4, although the coupling of per-benzoylated bromide 2b, prepared as shown in Scheme 2, with the sterically hindered acceptor 14 required prolonged reaction time (entries 4–6). These results were comparable with the glycosylations using traditional bromide activators - silver carbonate25,26 or mercury salts.27,28
Glycosidation of glycosyl thioimidates 3–5 was smoothly driven to completion in a matter of minutes (entries 7–16). All glycosylations proceeded with very high conversion yields, comparable, or even exceeding those achieved in glycosylations promoted with preactivated AgOTf. For example, AgBF4-promoted couplings depicted in entries 14 and 16 (Table 1) proceeded in 92% (90% with AgOTf) and 75% (70% with AgOTf) yield, respectively.9 While the anchimerically assisted couplings with benzoylated glycosyl donors were β-stereoselective, only fair α-stereoselectivity was achieved with 2-O-benzylated glycosyl donors. All coupling were performed in the neutral solvent since the stereoselectivity optimization was not primary intention of these studies.
Promoters for thioglycoside and n-pentenyl glycoside activation include thiophilic reagents, amongst which NIS/TfOH or NIS/TMSOTf are arguably the most common.29,30 Therefore, not surprisingly, the activation of stable glycosyl donors, ethyl thioglycoside 6 or n-pentenyl glycoside 7 did not take place in the presence of AgBF4. It should be noted that NIS alone can also activate both S-ethyl and pentenyl glycosides, however, these transformations require prolonged reaction time (entries 18 and 21). Having decided to investigate whether these glycosyl donors can be activated by NIS/AgBF4 system, we discovered that these coupling reactions proceeded very rapidly and provided the corresponding disaccharide in minutes (entries 19 and 22). We then demonstrated that AgBF4 could be employed as a suitable promoter for glycosidation of chloride 8, fluoride 9, or trichloroacetimidate 11, whereas the hemiacetal derivative 10 remained inert under these reaction conditions (entries 23–29).
Based on the results summarized in Table 1, we anticipated that it might be possible to activate glycosyl halides, S-benzothiazolyl, SBox, or STaz glycosides over the SEt or n-pentenyl moieties in the presence of AgBF4. We have already reported that selective activation of SBox and STaz glycosides over SEt glycosyl acceptor can be achieved in the presence of AgOTf.7,31 Also in this case, AgBF4 was found to be capable of providing smooth couplings with consistently high yields (Table 2). We observed that glycosidation of glycosyl fluoride 9 was sluggish and the moderate to good yields presented in Table 2 were based on the unreacted acceptor recovery (entries 4 and 9).
Table 2.
Selective activation of various glycosyl donors over n-pentenyl acceptor 20 and SEt acceptor 22
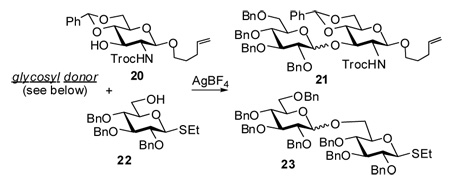 | ||||||
|---|---|---|---|---|---|---|
| entry | D | A | time | product | yield, % | α/β ratio |
| 1 | 3a | 20 | 10 min | 21 | 86 | 1.1/1 |
| 2 | 4a | 20 | 20 min | 21 | 85 | 3.3/1 |
| 3 | 5a | 20 | 10 min | 21 | 83 | 1/1.2 |
| 4 | 9 | 20 | 16 h | 21 | 65a | 1.6/1 |
| 5 | 2a | 22 | 15 min | 23 | 84 | 1.6/1 |
| 6 | 3a | 22 | 20 min | 23 | 87 | 2.0/1 |
| 7 | 5a | 22 | 15 min | 23 | 89 | 2.4/1 |
| 8 | 8 | 22 | 15 min | 23 | 84 | 2.2/1 |
| 9 | 9 | 22 | 24 h | 23 | 83a | 2.4/1 |
the yield is based on the recovered acceptor
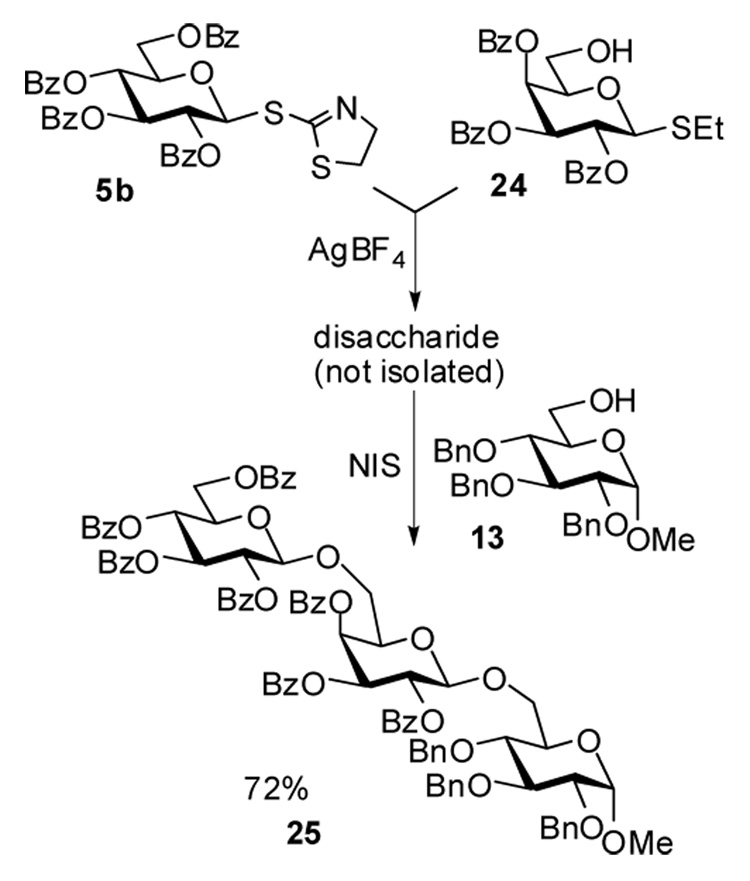
Based on the results of the selective activations, we assumed that it could be possible to perform the activation sequence in a highly efficient one-pot fashion. Presumably, any building block that can be activated with AgBF4 can be used as the glycosyl donor for the first activation step. We chose to investigate SBox and STaz glycosides as well as glycosyl bromides as glycosyl donors. Glycosyl acceptor should withstand AgBF4 activation, but in turn could be readily activated by addition of NIS. Either S-ethyl or O-pentenyl leaving groups could be used for this purpose. An example shown in Scheme 3 makes use of the STaz leaving group in 5b for the first coupling step. At this stage the S-ethyl moiety of the glycosyl acceptor 24 remains inert. Upon the formation of the intermediate disaccharide, its SEt moiety can be then activated for the reaction with the newly added acceptor 13 by adding 2.0 equiv. of NIS. As demonstrated above, NIS along with AgBF4 (used in excess and is remaining from the previous step) serves as an efficient promoter for this type of glycosylation. As a result of this two-step activation, trisaccharide 25 was isolated in 72% yield. Other leaving group combinations mentioned above were also found to be capable of the one-pot activations and provided the corresponding trisaccharides in 55–70% overall yields.
Scheme 3.
One-pot glycosylation: synthesis of trisaccharide 25.
In summary, we have identified silver tetrafluoroborate as an excellent promoter for the activation of various glycosyl donors, prepared from the common precursor bearing temporary STaz anomeric moiety. Easy handling and no requirement for azeotropic dehydration prior to the application makes AgBF4 especially beneficial in comparison to the commonly used AgOTf. We also demonstrated that selective activation of glycosyl halides or thioimidates over thioglycosides or n-pentenyl glycosides could lead to simple one-pot syntheses via sequential selective activation of one leaving group over another. Therefore, we believe that AgBF4 should be considered as a powerful and convenient alternative for silver(I) activated glycosylations.
Supplementary Material
Experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet
Supplementary Material
Acknowledgments
The authors thank the National Institutes of General Medical Sciences (GM077170) and the American Heart Association (AHA0660054Z) for financial support of this research; NSF for grants to purchase the NMR spectrometer (CHE-9974801) and the mass spectrometer (CHE-9708640) used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toshima K, Tatsuta K. Chem. Rev. 1993;93:1503–1531. [Google Scholar]
- 2.Davis BG. J. Chem. Soc., Perkin Trans. 2000;1:2137–2160. [Google Scholar]
- 3.Boons GJ. Contemp. Org. Synth. 1996;3:173–200. [Google Scholar]
- 4.Demchenko AV. Lett. Org. Chem. 2005;2:580–589. [Google Scholar]
- 5.El Ashry ESH, Awad LF, Atta AI. Tetrahedron. 2006;62:2943–2998. [Google Scholar]
- 6.Pornsuriyasak P, Kamat MN, Demchenko AV. ACS Symp. Ser. 2007;960:165–189. [Google Scholar]
- 7.Demchenko AV, Malysheva NN, De Meo C. Org. Lett. 2003;5:455–458. doi: 10.1021/ol0273452. [DOI] [PubMed] [Google Scholar]
- 8.Kamat MN, Rath NP, Demchenko AV. J. Org. Chem. 2007;72:6938–6946. doi: 10.1021/jo0711844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demchenko AV, Pornsuriyasak P, De Meo C, Malysheva NN. Angew. Chem., Int. Ed. 2004;43:3069–3072. doi: 10.1002/anie.200454047. [DOI] [PubMed] [Google Scholar]
- 10.Pornsuriyasak P, Demchenko AV. Chem. Eur. J. 2006;12:6630–6646. doi: 10.1002/chem.200600262. [DOI] [PubMed] [Google Scholar]
- 11.Pornsuriyasak P, Gangadharmath UB, Rath NP, Demchenko AV. Org. Lett. 2004;6:4515–4518. doi: 10.1021/ol048043y. [DOI] [PubMed] [Google Scholar]
- 12.Hanessian S, Banoub J. Carbohydr. Res. 1977;53:c13–c16. [Google Scholar]
- 13.Ziegler T, Kovac P, Glaudemans CPJ. Liebigs Ann. 1990;6:613–615. [Google Scholar]
- 14.Ronnow TECL, Meldal M, Book K. J. Carbohydr. Chem. 1995;14:197–211. [Google Scholar]
- 15.Szweda R, Spohr U, Lemieux RU, Schindler D, Bishop DF, Desnick RJ. Can. J. Chem. 1989;67:1388–1391. [Google Scholar]
- 16.Douglas SP, Whitfield DM, Krepinsky JJ. J. Carbohydr. Chem. 1993;12:131–136. [Google Scholar]
- 17.Mehta S, Pinto BM. J. Org. Chem. 1993;58:3269–3276. [Google Scholar]
- 18.Kronzer FJ, Schuerch C. Carbohydr. Res. 1973;27:379–390. [Google Scholar]
- 19.Tsui DSK, Gorin PAJ. Arquiv. Biol. Technol. 1985;28:575–580. [Google Scholar]
- 20.Verlhac P, Leteux C, Toupet L, Veyrieres A. Carbohydr. Res. 1996;291:11–20. [Google Scholar]
- 21.Lear MJ, Yoshimura F, Hirama M. Angew. Chem. Int. Ed. 2001;40:946–949. [PubMed] [Google Scholar]
- 22.Liu KKC, Danishefsky SJ. J. Org. Chem. 1994;59:1895–1897. [Google Scholar]
- 23.Suzuki K, Maeta H, Suzuki T, Matsumoto T. Tetrahedron Lett. 1989;30:6879–6882. [Google Scholar]
- 24.Toshima K. In: Glycoscience: Chemistry and Chemical Biology. Fraser-Reid B, Tatsuta K, Thiem J, editors. Vol. 1. Berlin - Heidelberg - New York: Springer; 2001. pp. 583–626. [Google Scholar]
- 25.Pozsgay V, Dubois EP, Pannell L. J. Org. Chem. 1997;62:2832–2846. doi: 10.1021/jo962300y. [DOI] [PubMed] [Google Scholar]
- 26.Ekelof K, Oscarson S. J. Org. Chem. 1996;61:7711–7718. doi: 10.1021/jo960789p. [DOI] [PubMed] [Google Scholar]
- 27.Pozsgay V. J. Org. Chem. 1998;63:5983–5999. doi: 10.1021/jo980660a. [DOI] [PubMed] [Google Scholar]
- 28.Pozsgay V. Angew. Chem., Int. Edit. 1998;37:138–142. [Google Scholar]
- 29.Oscarson S. In: Carbohydrates in Chemistry and Biology. Ernst B, Hart GW, Sinay P, editors. Vol. 1. New York: Wiley-VCH: Weinheim; 2000. pp. 93–116. [Google Scholar]
- 30.Fraser-Reid B, Anilkumar G, Gilbert MB, Joshi S, Kraehmer R. In: Carbohydrates in Chemistry and Biology. Ernst B, Hart GW, Sinay P, editors. Vol. 1. New York: Wiley-VCH: Weinheim; 2000. pp. 135–154. [Google Scholar]
- 31.Pornsuriyasak P, Demchenko AV. Tetrahedron: Asymmetry. 2005;16:433–439. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.