Abstract
Ovine pulmonary adenocarcinoma (OPA) is a naturally occurring lung cancer of sheep caused by Jaagsiekte sheep retrovirus (JSRV). The JSRV envelope glycoprotein (Env) functions as a dominant oncoprotein in vitro and in vivo. In order to develop the basis for the use of OPA as a lung cancer model, we screened a variety of signal transduction inhibitors for their ability to block transformation by the JSRV Env. Most inhibitors were not effective in blocking JSRV Env-induced transformation. On the contrary, various Hsp90 inhibitors efficiently blocked JSRV transformation. This phenomenon was at least partly due to Akt degradation, which is activated in JSRV-transformed cells. Hsp90 was found expressed in tumor cells of sheep with naturally occurring OPA. In addition, Hsp90 inhibitors specifically inhibited proliferation of immortalized and moreover primary cells derived from OPA tumors. Thus, OPA could be used as a large animal model for comprehensive studies investigating the effects of Hsp90 inhibitors in lung adenocarcinoma.
INTRODUCTION
The understanding of the molecular mechanisms governing pulmonary oncogenesis has increased tremendously throughout the last decade (Minna, Roth, and Gazdar, 2002). However, lung cancer is still the most common cause of death of cancer patients worldwide and its survival rate after 5 years is extremely poor, highlighting the urgent need for the development of better therapies and early detection strategies (Parkin et al., 2005). To this end, appropriate animal models can be of great help in understanding the molecular basis of lung cancer, designing candidate therapeutic interventions, new surgical procedures and testing novel imaging technologies for early diagnosis.
A variety of mouse models are available for lung cancer (Dutt and Wong, 2006). Transgenic and especially “conditional” mouse models, had a dramatic effect in understanding the contribution of oncogenes in the onset and maintenance of cancer (Varmus et al., 2005). In the pre-clinical settings, treatment of xenograft mouse models is routinely the first step used to test new anticancer drugs. However, most anticancer drugs fail in phase I and II clinical trials (Rothenberg, Carbone, and Johnson, 2003).
Neoplasms of domestic animals are not extensively used as cancer models. The large body of knowledge in mouse genetics, the possibility to manipulate their genome and the availability of biological reagents make rodents the natural choice as disease model organisms. Large and domestic animals are more difficult and generally more expensive to manage compared to mice or rats. However, the completion of the sequencing of the genome of several domestic animal species and the development of new cloning and transgenic techniques open the possibility to explore other animal species as cancer models (Khanna et al., 2006). Ovine pulmonary adenocarcinoma (OPA) is a naturally occurring lung cancer of sheep (Fan, 2003) caused by a retrovirus known as Jaagsiekte sheep retrovirus (JSRV) (Palmarini and Fan, 2003; Palmarini et al., 1999). Among retroviruses, JSRV follows unique mechanisms to induce cell transformation, since its envelope (Env) glycoprotein functions as a dominant oncoprotein both in vitro (Allen et al., 2002; Maeda et al., 2001; Palmarini and Fan, 2003; Palmarini et al., 1999; Rai et al., 2001) and in vivo (Caporale et al., 2006; Wootton, Halbert, and Miller, 2005). The molecular mechanisms underlying JSRV Env-induced transformation have not been fully characterized but several pieces of evidence point to the involvement of the Ras-MEK-MAPK and PI3K-AKT pathways (De Las Heras et al., 2006; Maeda et al., 2005; Palmarini et al., 2001).
OPA shares many similarities with some forms of human lung adenocarcinomas (Mornex et al., 2003; Palmarini and Fan, 2001). In addition, OPA has several features suggesting that it can be developed into a useful animal model for lung cancer: (i) sheep and humans have a comparable lung size and tumor to body mass ratio; (ii) tumors in OPA can grow for a long time in the presence of a functional immune system; (iii) the disease is experimentally reproducible (Palmarini et al., 1999; Sharp et al., 1983) and the location/extent of the induced lesions can be modulated by using replication defective viruses delivered to specific sites with an intrabronchial delivery (Caporale et al., 2006).
The aim of this study was to identify signalling pathways involved in JSRV mediated transformation and to establish the basis for the use of OPA as a model to study the effects of small molecule inhibitors in cancer development. We provide data showing that several Hsp90 inhibitors efficiently block transformation of rodent fibroblasts by the JSRV Env and revert the phenotype of cells already transformed by this oncoprotein. This phenomenon was due at least in part to Akt degradation, which is normally activated in JSRV-mediated transformation (Caporale et al., 2006; Palmarini et al., 2001). Importantly, Hsp90 was found expressed in tumor cells of sheep with naturally occurring OPA and Hsp90 inhibitors reduced proliferation of primary and immortalized cell lines derived from OPA tumors. Targeting of the Hsp90 molecular chaperone has great potential for cancer therapy (Workman, 2004). Thus, OPA could be used as a large animal model for comprehensive studies investigating the effects of Hsp90 inhibitors.
RESULTS
Effects of signal transduction inhibitors in JSRV-induced cell transformation of rodent fibroblasts
Our first goal was to identify inhibitors of signal transduction pathways that efficiently blocked JSRV Env-induced cell transformation.
We assessed a total of 22 inhibitors, each of them in two different experimental settings. In the first series of experiments, we used a cell line transformed by the JSRV Env (208F-tr) and determined whether the addition of various inhibitors reverted the phenotype of the transformed cells to the parental cell line. Each inhibitor was used at least at two different concentrations ranging from 1 to 10 times its reported IC50. The highest concentration of each inhibitor that did not induce cell toxicity was used in standard transformation assays performed in the 208F cell line. In these series of experiments, cells were transfected with an expression plasmid for the JSRV Env and cultured in the presence or absence of each inhibitor. Foci of transformed cells were counted 15 days post-transfection. Each experiment was repeated at least twice. Results obtained are summarized in Table 1.
Table 1.
Effect of inhibitors on JSRV induced cell transformation of 208F cells
| Pathway | Inhibitor name | Inhibitor concentration (uM) | Inhibition of transformationa (%) | Reversion of transformed phenotype |
|---|---|---|---|---|
| Janus protein tyrosine kinase | JAK inhibitor I | 0.001 | n.a.b | No |
| 0.005 | n.a. | No | ||
| 0.01 | n.a. | No | ||
| 0.015 | n.a. | No | ||
| 0.05 | n.a. | No | ||
| 0.15 | 0 | No | ||
| VEGFR | VEGF receptor 2 kinase inhibitor III | 1 | 0 | No |
| 20 | n.a. | Txc | ||
| SRC (family) | PP2 | 5 | 41 | No |
| 10 | 73.4 | No | ||
| PP1 | 0.0015 | No | ||
| 0.015 | 0 | No | ||
| Genistein | 25 | 11.9 | No | |
| Inhibitor I | 0.044 | n.a. | No | |
| 0.088 | n.a. | No | ||
| 0.44 | n.a. | No | ||
| 0.88 | 0 | No | ||
| Inhibitor II | 1.2 | 0 | No | |
| 12 | TX | Tx | ||
| Emodin | 0.018 | n.a. | No | |
| 18.5 | 0 | No | ||
| 185 | n.a. | Tx | ||
| SU 6656 | 0.28 | n.a. | No | |
| 1 | 22 | n.a. | ||
| 2.8 | 65.9** | Yes | ||
| Lavendustin C | 2 | 4 | No | |
| Damnacanthal | 0.017 | n.a. | No | |
| 0.17 | 0 | No | ||
| Platelet derived growth factor receptor (PDGF) | Inhibitor I | 0.2 | n.a. | No |
| 2 | n.a. | No | ||
| Inhibitor II | 1.1 | n.a. | No | |
| 11 | 57.5 | No | ||
| Inhibitor III | 0.05 | n.a. | No | |
| 0.08 | 20 | No | ||
| 0.5 | n.a. | No | ||
| 0.8 | 0 | No | ||
| AG 1296 | 1 | n.a. | No | |
| 10 | n.a. | No | ||
| AG 2043 | 1 | n.a. | No | |
| 10 | 29.2 | No | ||
| EGFR | PD 153035 | 0.000025 | n.a. | No |
| 0.00025 | 0 | No | ||
| AG 1478 | 0.003 | n.a. | No | |
| 0.03 | 0 | No | ||
| HSP 90 | Herbimycin A | 0.2 | 96 | n.a. |
| 0.4 | 100 | Yes | ||
| Geldanamycin | 0.0017 | 0 | n.a. | |
| 0.0075 | 74 | n.a. | ||
| 0.0187 | 90 | n.a. | ||
| 0.0375 | 97.9 | Yes | ||
| 0.075 | Tx | Yes | ||
| 0.75 | Tx | Yes | ||
| Radicicol | 0.27 | n.a. | Yes | |
| 2.7 | 55.7 | Yes | ||
| 17-DMAG | 0.5 | 88.8 | Yes | |
Inhibition of transformation was calculated by comparing the number of foci obtained by the JSRV Env in the presence of each inhibitor or in the presence of DMSO alone (taken as 100%). Values shown are the average of at least two experiments.
Not analysed
A toxic effect was evident at these concentrations
Inhibitors against the Janus protein kinase (JAKs), vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) did not affect transformation by the JSRV Env since no or minimal reduction in the number of foci was observed in cultures treated with inhibitors compared to the control ones treated with DMSO. Inhibitors against platelet-derived growth factor receptor (PDGF) reduced the number of transformed foci induced by the JSRV Env from 30 to 60% as compared with cells treated with DMSO alone. However, the PDGF inhibitors used had a noticeable toxic effect in 208F cells and consequently the reduction in the number of transformed foci could be due simply to this phenomenon. Neither the PDGF inhibitors nor the inhibitors mentioned above were able to revert the phenotype of 208-tr. These data indicate that signalling through the JAKs, VEGF receptor, PDGF receptor and EGFR do not play a major role in JSRV induced cell transformation of rodent fibroblasts.
Src contributes to JSRV Env-induced cell transformation
As shown in Table 1, seven of nine inhibitors against the Src family of non receptor tyrosine kinases neither reverted the phenotype of 208F-tr cells nor reduced the number of transformed foci in standard JSRV Env transformation assays. However, SU6656 reverted the transformed phenotype of 208F-tr cells to a flatter and less translucent morphology and slightly reduced transformation. In addition, when transformation assays were performed in the presence of PP2 the number of foci of transformed cells induced by the JSRV Env was drastically reduced (∼70%).
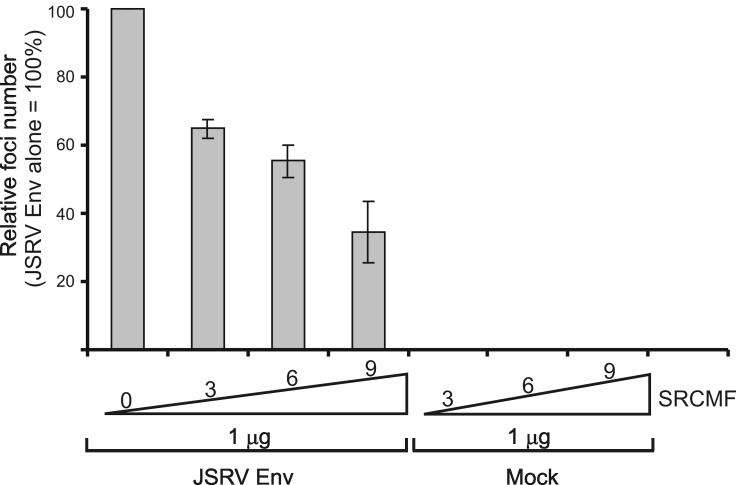
The differences on the effects seen among the various Src inhibitors are not surprising since the specificity and potency towards each Src family member varies (Blake et al., 2000; Karni et al., 2003). In addition, PP2 was shown previously to have an effect on JSRV Env-induced cell transformation (Hull and Fan, 2006). To further understand the role of Src in JSRV Env mediated transformation we co-transfected 208F cells with the expression plasmid for the JSRV Env and increasing amounts of a dominant negative form of Src (SrcMF) (Timpson et al., 2001). As shown in Figure 1, we found a dose dependent inhibition of JSRV Env-induced transformation by SrcMF. As a whole the data described above suggest that Src may be partially involved in the mechanisms of JSRV Env-induced cell transformation.
Fig. 1. Src is involved in part in JSRV Env induced cell transformation.
208F cells were co-transfected with 1 μg of an expression plasmid for the JSRV Env and increasing amounts of a plasmid expressing a dominant negative form of Src (SrcMF). Results show the average of two independent experiments and are expressed as a percentage of transformation where the number of foci resulting from transfection of 1 μg of JSRV Env alone is considered 100%. Bars represent standard deviation.
Hsp90 inhibitors block transformation by the JSRV Env
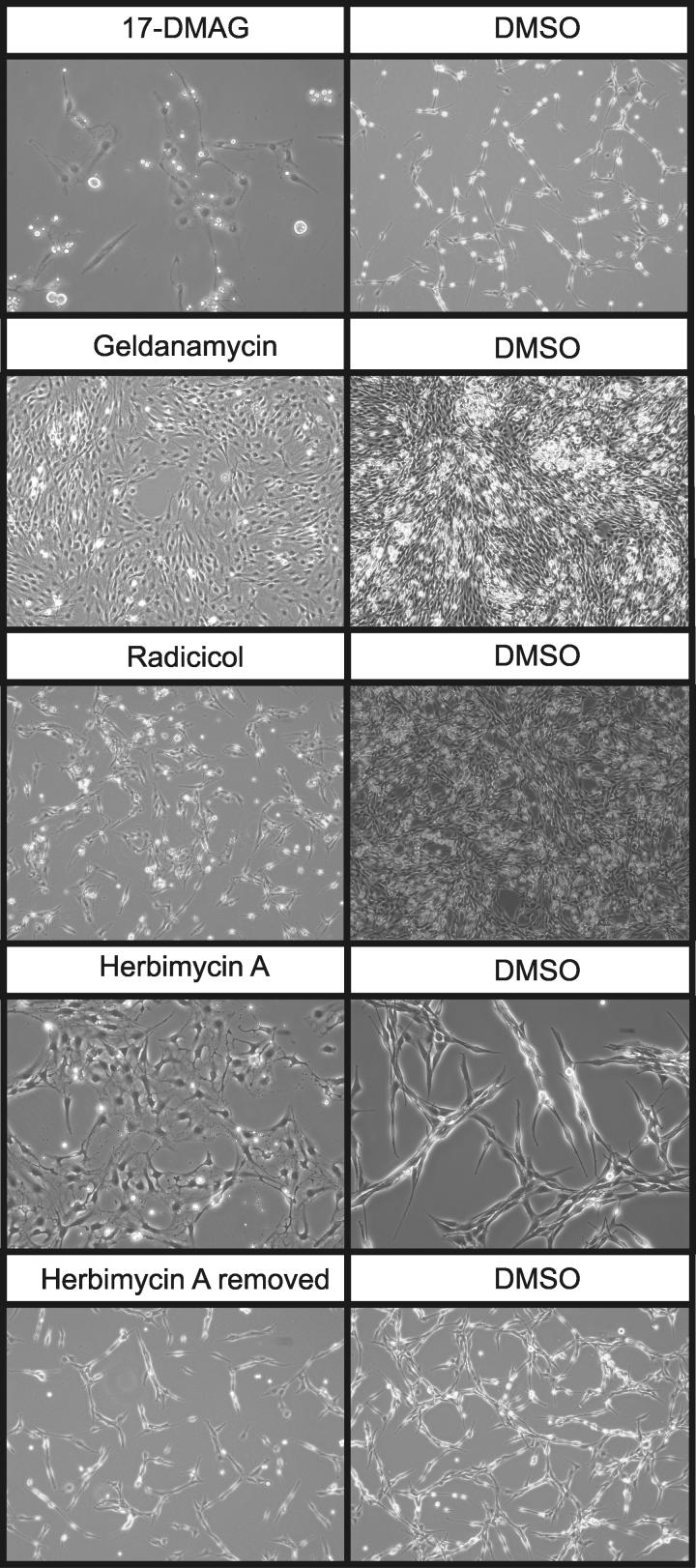
We next examined several Hsp90 inhibitors including herbimycin A (HA), geldanamycin (GA), radicicol and 17-DMAG. All the above inhibitors suppressed transformation in a dose-dependent manner (Table 1) and reverted the transformed phenotype of 208F-tr cells to a flatter and less translucent morphology compared to control 208-tr cells (Fig. 2). Once the drugs were removed from the culturing media, cells returned to display their original transformed phenotype demonstrating that the drugs had no effect on integration and expression of the JSRV Env plasmid (Fig. 2 bottom panel). These results indicate that Hsp90 is involved in the initiation and progression of the transformation process mediated by the JSRV Env as well as in the maintenance of the transformed phenotype in vitro.
Fig. 2. Reversion of transformed phenotype of 208F-tr cells.
208-tr cells derive from a focus of cells transformed by the JSRV Env. These cells were cultured in the presence of Hsp90 inhibitors (or DMSO as negative control) and their morphology was monitored for 5 days. Illustrative examples of 208-tr cells cultured at higher or lower density in the presence of Hsp90 inhibitors are shown.
Hsp90 is a molecular chaperone that participates in the folding, assembly, maturation and stabilization of “client” proteins including a variety of signalling molecules and transcription factors that are crucial for oncogenesis such as AKT, HER2, c-SRC, NFκB, IGFR1, p53 and RAF among others. Consequently, Hsp90 inhibitors are promising therapeutic drugs (Blagg and Kerr, 2006).
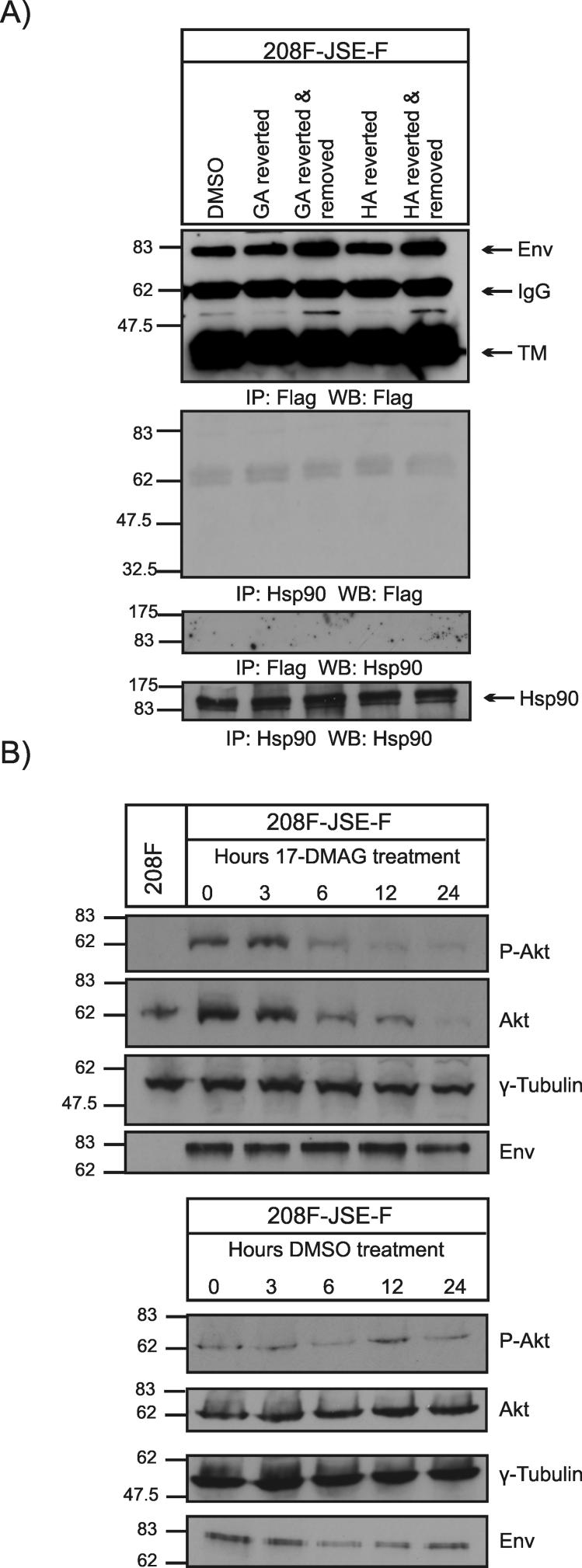
To further understand the mechanisms underlying the effects of Hsp90 inhibitors in JSRV-transformed cells, we examined whether the JSRV Env was an Hsp90 client protein. If this was the case, the block in transformation and the reversion of the transformed phenotype seen with the various Hsp90 inhibitors would be due to association of Hsp90 with the JSRV Env followed by proteasomal degradation. To this end, we assessed the expression of the JSRV Env by western blotting in total cell lysates extracted from transformed 208F-tr cells or from 208F-tr cells that reverted to a flatter morphology in the presence of Hsp90 inhibitors (Fig. 3A). We could not detect down-regulation of the JSRV Env in 208F-tr cells when the phenotype was reverted to a more flat morphology in the presence of GA or HA. Moreover, we did not find association between the JSRV Env and Hsp90 by co-immunoprecipitation assays strongly suggesting that the JSRV Env is not an Hsp90 client protein (Fig. 3A).
Fig. 3. Influence of Hsp90 inhibitors on JSRV Env and Akt expression.
A. Total cell extracts were obtained from 208F-tr cells in the presence of DMSO (negative control) or from 208-tr that had reverted their transformed phenotype in the presence of geldanamycin (GA) or herbimycin A (HA). In addition, cell extracts were also obtained from 208F-tr that returned to the transformed phenotype once GA or HA were removed from the culturing media. 200 μg of cell extracts were immunoprecipitated and analysed by western blotting as indicated below each panel. Note that the JSRV Env expressed in 208F-tr is tagged with a FLAG epitope at its C terminus. TM indicates the transmembrane domain of the JSRV envelope (Env) glycoprotein. B. 208F-tr cells were cultured in serum free media with the addition of 17-DMAG or DMSO as a negative control for 3, 6, 12 and 24 h. At each time point cells were lysed and analysed by western blotting for the presence of Akt and pAkt. Detection of γ-tubulin was used as loading control.
Hsp90 inhibitors induce Akt degradation
Akt is an Hsp90 client protein and the association between Hsp90 and Akt modulates the kinase activity of the latter (Sato, Fujita, and Tsuruo, 2000). Akt activation plays an important role in JSRV Env-mediated transformation of 208F cells (Chow et al., 2003; Liu, Lerman, and Miller, 2003; Palmarini et al., 2001). Thus, we tested whether changes in the expression (or phosphorylation status) of Akt could be the cause of the effects of the Hsp90 inhibitors on JSRV Env-induced transformation, since the Env itself is not an Hsp90 client protein. To address this point, we cultured 208F-tr cells in serum free media with the addition of 17-DMAG (0.5 μM) (or DMSO as control) for a period of 3, 6, 12 and 24 hours. Thereafter, total cell lysates were analysed by western blotting. We observed time dependent Akt degradation and dephosphorylation at serine 473 when cells were cultured with 17-DMAG while no changes were seen in the expression of the JSRV Env or γ-tubulin that was used as loading control (Fig. 3B). No changes in the phosphorylation status or expression of Akt or the JSRV Env were observed and no changes in the transformed morphology of these cells were noticeable when cells were cultured with DMSO as a control. Akt degradation was observed when the same experiment was performed in the presence of radicicol, while no changes were noticeable in the level of expression of the JSRV Env or γ-tubulin (not shown). These data indicate that the reversion of the transformed phenotype seen with the Hsp90 inhibitors could be due at least in part to the degradation of Akt.
Hsp90 is expressed in OPA tumor cells in vivo
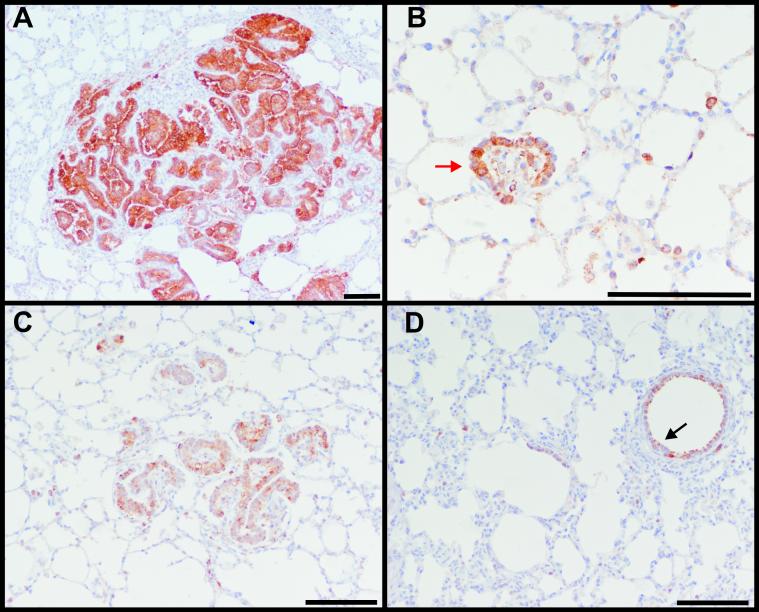
Above, we demonstrated that Hsp90 inhibitors are able to block transformation of rodent fibroblasts (and revert the phenotype of cells already transformed) by the JSRV Env with a mechanism dependent, at least in part, on Akt degradation. Here, we assessed whether Hsp90 is expressed in OPA tumors, in order to determine whether the data obtained in rodent fibroblasts in vitro could eventually be translated into the JSRV/OPA model in vivo. Lung sections from tumors of 3 sheep with naturally occurring OPA and 3 with experimentally-induced disease were analyzed by immunohistochemistry using antibodies towards the JSRV Env (to verify JSRV expression) or Hsp90. As expected, the JSRV Env was expressed in the lung tumor cells of animals with OPA (Fig. 4A). Hsp90 was found to be highly expressed in tumor cells of both small and more advanced lesions (Fig. 4B-C) although Hsp90 expression was also detected in normal bronchiolar, alveolar and interstitial cells of both OPA and healthy sheep (Fig. 4C-D).
Fig. 4. Hsp90 is expressed in OPA tumor cells.
Immunohistochemistry in lung sections from naturally occurring OPA cases show expression of the JSRV Env in tumor cells (characterized by the intracytoplasmic brown color) (A) and Hsp90 (B-D). Hsp-90 is found expressed mainly in the tumor cells. Expression of Hsp90 in epithelial cells outside the tumor lesions and moreover in the bronchiolar cells in lung sections from both OPA (B-C) and healthy sheep (D, indicated by the black arrow) was also observed. In panel B, the red arrow indicates tumor cells in an early neoplastic lesion. Bars represent 100 μm.
Hsp90 inhibitors reduce proliferation of OPA-derived immortalized and primary cell lines
In order to better assess the effects of Hsp90 inhibitors on JSRV-induced transformation we analyzed their effects on the growth of tumor cells derived from OPA lesions. Firstly, we used primary tumor cells from naturally occurring OPA cases and primary type II pneumocytes from healthy sheep as control cultures. Normal type II pneumocytes were found to express markers such as SP-A, SP-C and presented lamellar bodies by electron microscopy (Archer et al., 2007). Tumor cells were confirmed to express JSRV by the detection of reverse transcriptase activity in the culture supernatants and the detection of the viral major capsid protein by western blotting (Archer et al., 2007).
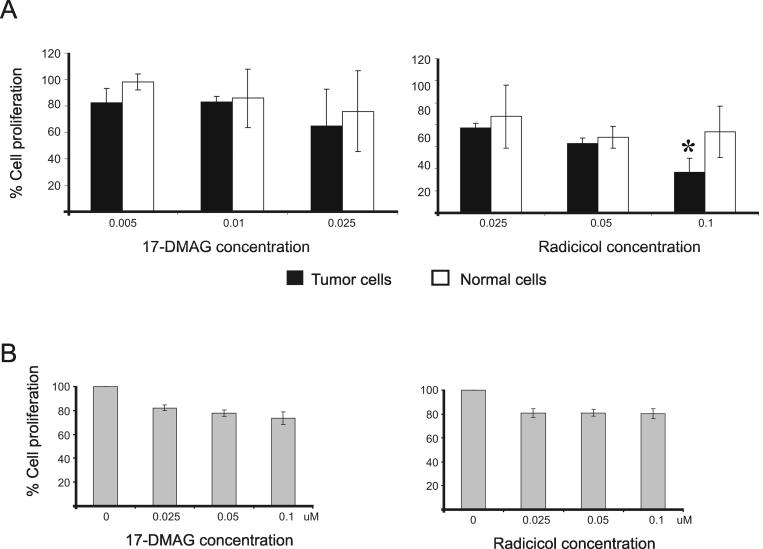
Normal and transformed alveolar type II cells were grown in the presence or absence of increasing amounts of radicicol or 17-DMAG for 48 hours and their proliferation was assessed as described in Materials and Methods. We found a significant reduction (p=0.04) in the growth of tumor cells as compared to the normal type II pneumocytes in the presence of 0.1 μM of radicicol while the effects of 17-DMAG were more variable (Fig. 5A).
Fig. 5. Hsp90 inhibitors reduce proliferation of OPA derived cells.
A.17-DMAG equally affects the growth rate of tumoral and normal type II pneumocytes while radicicol specifically reduces proliferation of the tumor cells (p=0.04). B. 17-DMAG and radicicol significantly reduce (p 0.0002) proliferation of JS8 cells which is an immortalized cell line derived from a lung tumor of a sheep affected by OPA. Bars in both panels represent the standard deviation.
Secondly, we analyzed the effects of Hsp90 inhibition in JS8 cells which is an immortalized cell line derived from a lung tumor of a sheep affected by OPA (Jassim, 1988; Jassim, Sharp, and Marinello, 1987). Cells were grown for 72 hours in the presence of increasing amounts of radicicol and 17-DMAG. We found statistically significant inhibition (p=0.0002) in cell proliferation when cells were grown in the presence of 17-DMAG and radicicol at all the concentrations tested (Fig. 5B). Thus at least radicicol can block proliferation of OPA tumor cells.
DISCUSSION
The aim of this study was to identify signalling pathways involved in JSRV induced cell transformation by the use of drugs that could efficiently block transformation by the JSRV Env in vitro and to establish the functional basis for the development of OPA as a large animal model for lung cancer. JSRV is unique among oncogenic retroviruses because its envelope glycoprotein functions as a dominant oncoprotein (Allen et al., 2002; Maeda et al., 2001; Palmarini and Fan, 2003; Palmarini et al., 1999; Rai et al., 2001). Transfection of a variety of cell lines with expression plasmids for the JSRV Env readily results in the induction of foci of transformed cells. In addition, adeno-associated viral vectors expressing the JSRV Env induce lung cancer in immunosuppressed mice (Wootton, Halbert, and Miller, 2005). Furthermore, replication defective JSRV vectors expressing only the viral Env induce lung cancer in sheep, the natural host of JSRV infection (Caporale et al., 2006). Thus, the JSRV/OPA model is an excellent system where the significance of findings obtained in vitro can be immediately translated in vivo.
We found that the molecular chaperon Hsp90 is involved in the mechanisms of cell transformation induced by the JSRV Env. Indeed, various Hsp90 inhibitors efficiently blocked transformation in vitro by the JSRV Env and reverted the morphology of cells already transformed by it. In addition, we demonstrated that (i) Hsp90 is expressed in OPA tumor cells and (ii) proliferation of OPA-derived tumor cells is inhibited by radicicol. The reduction of the proliferation of OPA tumor cells after drug treatment was modest but this could be due to a somewhat reduction in the transformed phenotype of the primary tumor cells considering that JSRV expression decreases over time with the passaging of these cells (Archer et al., 2007). Also the JS8 cell line has been passaged extensively and does not release JSRV viral particles in the supernatants (data not shown).
Thus, OPA could be used as an alternative large animal model for the development of Hsp90 inhibitors and the study of the molecular mechanisms underlying their effects in cancer development. The JSRV Env is not an Hsp90 client protein considering that (i) Hsp90 and the JSRV Env do not co-immunoprecipitate and (ii) Hsp90 inhibitors do not affect the levels of expression of the JSRV Env in 208-tr cells reverted to a flatter untransformed morphology. Hsp90 inhibitors reduced the levels of Akt expression in 208F cells transformed by the JSRV Env. Activation of the PI3K/Akt pathway is one of the features displayed by cells transformed by the JSRV Env and the inhibitory effects of the Hsp90 inhibitors in this system could be due, at least in part, to Akt degradation.
Lung cancer is a multi-step process that involves the accumulation of genetic and epigenetic alterations that cause the activation of several signal pathways simultaneously (Digel and Lubbert, 2005; Girard et al., 2000). Ideally, therapeutic interventions for cancer should be able to interfere with a variety of signal transduction pathways that are involved in cell transformation. Heat shock proteins have been found to be overexpressed in several haematological and solid human cancers, including lung cancer (Chant, Rose, and Morris, 1995; Santarosa et al., 1997; Senju et al., 2006; Yufu, Nishimura, and Nawata, 1992). For reasons that yet remain to be fully clarified, Hsp90 extracted from tumor cells has a higher binding affinity for 17-AAG than Hsp90 extracted from normal tissue, allowing the accumulation of the drug in tumors (Kamal et al., 2003). Moreover, Hsp90 inhibitors have been shown to reduce proliferation of several human lung cancer cell lines and induce further growth inhibition when combined with irradiation (Senju et al., 2006). The ability of Hsp90 inhibitors to disrupt a variety of signalling pathways that are involved in the development of cancer makes them ideal therapeutic agents for the treatment of lung cancer.
The mechanisms of cell transformation by the JSRV Env are not completely clarified but involve the PI3K-Akt, the Ras-MEK-MAPK pathways and possibly, as shown in this study, also Src considering that two Src inhibitors and a dominant negative Src (SrcMF) reduced JSRV Env transformation. All these pathways have been implicated in the development of human lung cancer (Brognard et al., 2001; Kolch, 2002; Vivanco and Sawyers, 2002; Zhang et al., 2007). Thus, JSRV-mediated transformation can be a useful model to study the molecular mechanisms underpinning the effects of Hsp90 inhibitors on particular cell signalling molecules in tumors where several pathways are activated simultaneously, both in vitro and in vivo.
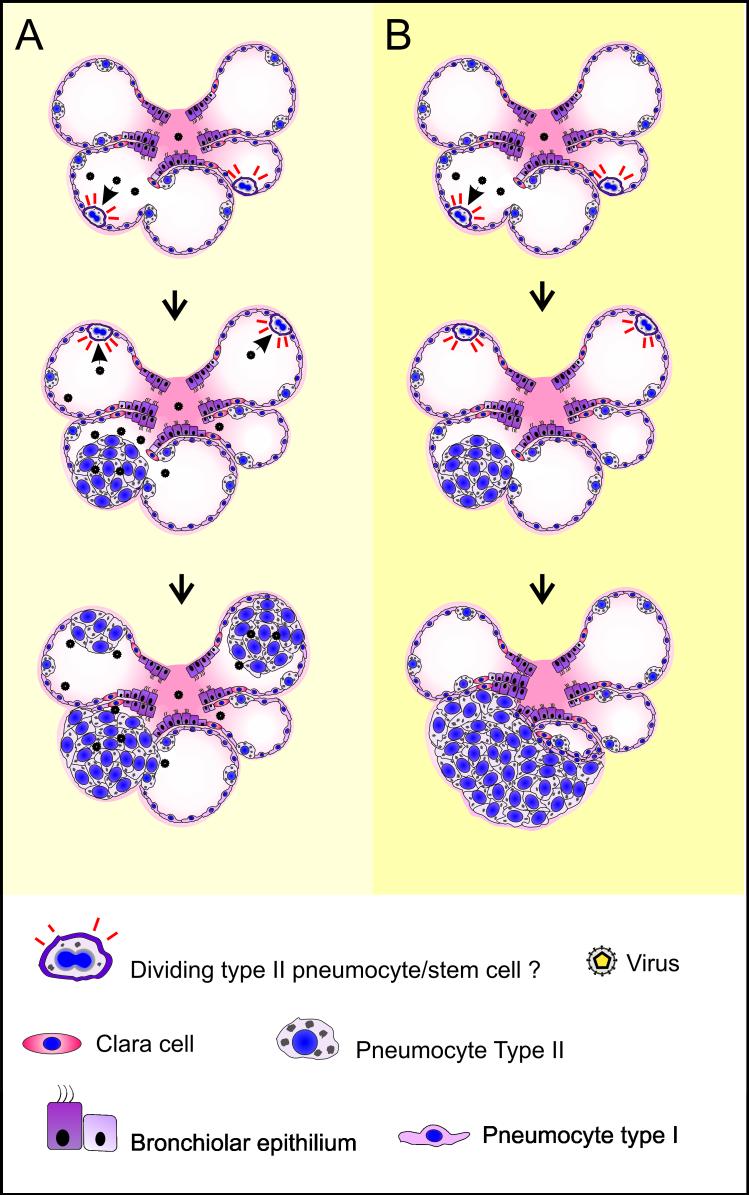
There is an increasing need of animal models for studying the safety and efficacy of the numerous anticancer drugs that are under development (Collis, 2006). OPA can be experimentally reproduced with a short incubation period (∼4-12 weeks) when lambs are inoculated intratracheally with concentrated virus preparations (Fig. 6). Under these circumstances, the primary target cells of infection produce new infectious virus that is able to infect and hence transform new cells leading to the appearance of lesions of different sizes that tend to coalesce. It could be argued that the use of this model could be “overpowering” even for effective drugs, given that new infectious virus expressing a dominant oncoprotein is continuously produced. However, we recently developed a JSRV-replication-defective virus (JS-RD) (Caporale et al., 2006) that proved to be oncogenic in a high percentage of inoculated lambs. In addition, JS-RD can be inoculated by bronchoscopy in well defined anatomical regions of the lungs, increasing the opportunity to develop intravitam imaging techniques where lesion development is continuously monitored.
Fig. 6. Extension of the OPA lesions can be modulated by the use of replication competent or incompetent JSRV viruses.
Cartoon representing two experimental models of OPA. A. Young lambs have many available target cells (pneumocytes type II or possibly pulmonary stem cells) that can be infected and transformed by JSRV. In turn, the neoplastic cells produce infectious virus that can then infect and transform other target cells resulting in many satellite and coalescing lesions. On the other hand, experimental inoculation of young lambs with the replication incompetent JS-RD virus (panel B) will result in infection and transformation of target cells that do not produce infectious virus, resulting in tumor nodules derived from a single transformed cell. The cartoon is only a schematic representation of the histopathological lesions in OPA. Tumor cells in OPA grow usually in a well organized manner along the alveolar walls.
The finding that the effects of inhibitors of Hsp90 in cell transformation can be studied in this system demonstrates that OPA could be used as tool for the development and improvement of other Hsp90 inhibitors. Although animals affected by OPA have not been used to test the therapeutic potential of any drugs so far, inhibitors of Hsp90 offer an interesting opportunity to challenge OPA in this regard considering the promising in vitro findings shown in this study. In conclusion, OPA could be used as a model where integrated approaches and protocols including imaging for early diagnosis, chemotherapy, radiotherapy and surgery could be experimented and developed. In this respect, OPA (and in general large animal cancer models) can be a valid alternative to rodent models.
MATERIALS AND METHODS
Inhibitors
All inhibitors used in this study were purchased from Calbiochem. The inhibitors and their concentration of use are listed in Table 1.
Cells and transformation assays
208F cells were grown in Dulbecco’s modified Eagle’s medium with high glucose (4.5 g/l) supplemented with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere and 95% humidity. Transformation assays were performed by transfecting 5 × 105 208F cells (per 10 cm diameter plate) with pCMV3JS21ΔGP (Maeda et al., 2003; Palmarini et al., 1999; Rai et al., 2001), an expression plasmid of the JSRV Env or an empty vector using Calphos mammalian transfection kit (Clontech) following the manufacturer’s instructions. Cells were washed 12-16 hours after transfection with phosphate-buffered saline (PBS) and split into 4 × 6 cm plates. Cell culture medium was replaced every other day for one week with the addition of 1 μM of dexamethazone. Thereafter, two cell culture dishes were treated with inhibitor and the remaining two with DMSO as negative control. Foci of transformed cells were counted 14 days post transfection and ranged between zero and 300 per dish depending on the degree of inhibition of transformation. Transformation assays with a dominant negative form of Src (Timpson et al., 2001) were performed by transfecting 1 μg of pCMV3JS21ΔGP and increasing amounts of SrcMF (kindly provided by Valerie Brunton). Foci of transformed cells were counted 14 days post transfection.
To monitor the effects of various signal transduction inhibitors on cells already transformed by the JSRV Env, we used 208F-tr cells. 208F-tr derive from a focus of 208F cells transformed by JSRV Env tagged with a FLAG epitope. 208F-tr were allowed to reach 60% confluence before inhibitors (or DMSO) were added to the media for five days.
OPA-derived immortalized and primary cell lines
Ovine primary alveolar type II cells from healthy sheep or tumor cells from sheep with OPA were isolated, cultured and characterized as described previously (Archer et al., 2007). Briefly, primary cells were cultivated in the selective epithelial medium Quantum 286 (PAA, Pasching, Austria) complemented with keratinocyte growth factor (KGF 10ng/ml, Abcys), hepatocyte growth factor (HGF 5ng/ml, Abcys), penicillin/streptomycin and cultured in 5% CO2 at 37°C. Tumor cells derived from OPA tumors presented a proliferative advantage compared to cells derived from normal lungs as observed previously (Suau et al., 2006).
Normal and tumor alveolar type II cells were plated in 96 wells plates (5× 102 cells/ well) and cultured for 48 hours in the presence of radicicol or 17-DMAG. Thereafter cell proliferation was measured using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Charbonnières, France). Experiments were repeated independently three times with at least two replicates per each experiment. Data was analyzed using a two-way ANOVA test.
JS8 is an immortalized cell line derived from lung tumors of a sheep with naturally occurring OPA (Jassim, 1988; Jassim, Sharp, and Marinello, 1987). JS8 cells were plated in 96 well dishes at a density of 103 cells/well and grown in F12-DMEM media supplemented with 10% of FBS with or without the addition of radicicol or 17-DMAG for 72 hours. Cell proliferation was measured using the WST-1 assay (Roche) following the instructions of the manufacturer and data was analyzed using an unpaired t-test.
Antibodies
Antibodies for AKT and phosphorilated AKT (S473) were purchased from Cell Signalling. Monoclonal anti-Flag M2 antibodies were purchased from Sigma. Hsp90 antibodies were purchased from Santa Cruz Biotechnology. Secondary anti-rabbit IgG peroxidase linked F(ab’) fragment from donkey was purchased from Amersham Biosciences. Peroxidase conjugated goat anti-mouse antibodies were purchased from Jackson Research.
Co-immunoprecipitation assays
Cells were lysed with SDS-NP-40 lysis buffer [0.5% Sodium Deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 0.5% NP-40, 50 mM TRIS, 150 mM NaCl, protease inhibitors cocktail (Complete-Roche) and 1 mM of PMSF] or with a milder lysis buffer (20 mM Hepes, 150 mM NaCl, 0.5 % beta maltoside and 0.5% of Triton-X) and immunoprecipitated and analysed by western blot as previously described (Varela et al., 2006).
Immunohistochemistry
4-6 μm lung sections from healthy sheep (n=2), lambs with experimentally induced OPA (n=3) or sheep with naturally occurring OPA (n=3) were stained with haematoxylin and eosin and examined by light microscopy for tumor lesions. Tumors were confirmed to be caused by JSRV by immunohistochemistry using antibodies towards the JSRV Env or the JSRV matrix (MA) as previously described (Caporale et al., 2006; Salvatori et al., 2004). Expression of Hsp90 in OPA tumor cells was investigated by using anti Hsp90 antibodies (Santa Cruz.). The EnVision (Dako) visualization system was used for both the detection of JSRV proteins and Hsp90.
Acknowledgements
We would like to thank Valerie Brunton for plasmid SrcMF and useful suggestions, Marco Caporale for providing Fig. 6 and Pablo Murcia for advice. This work was funded by grant CA95706-01 from the National Cancer Institute of the National Institutes of Health. MP is a Royal Society Wolfson Research Merit Awardee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen TE, Sherrill KJ, Crispell SM, Perrott MR, Carlson JO, DeMartini JC. The jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J Gen Virol. 2002;83(Pt 11):2733–2742. doi: 10.1099/0022-1317-83-11-2733. [DOI] [PubMed] [Google Scholar]
- Archer F, Jacquier E, Lyon M, Chastang J, Cottin V, Mornex JF, Leroux C. Alveolar Type II Cells Isolated from Pulmonary Adenocarcinoma: A Model for JSRV Expression in vitro. Am J Respir Cell Mol Biol. 2007;36(5):534–540. doi: 10.1165/rcmb.2006-0285OC. [DOI] [PubMed] [Google Scholar]
- Blagg BS, Kerr TD. Hsp90 inhibitors: small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med Res Rev. 2006;26(3):310–38. doi: 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20(23):9018–27. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61(10):3986–97. [PubMed] [Google Scholar]
- Caporale M, Cousens C, Centorame P, Pinoni C, De las Heras M, Palmarini M. Expression of the Jaagsiekte sheep retrovirus envelope glycoproteins is sufficient to induce lung tumor in sheep. Journal of Virology. 2006;80:8030–8037. doi: 10.1128/JVI.00474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant ID, Rose PE, Morris AG. Analysis of heat-shock protein expression in myeloid leukaemia cells by flow cytometry. Br J Haematol. 1995;90(1):163–8. doi: 10.1111/j.1365-2141.1995.tb03395.x. [DOI] [PubMed] [Google Scholar]
- Chow YH, Alberti A, Mura M, Pretto C, Murcia P, Albritton LM, Palmarini M. Transformation of rodent fibroblasts by the jaagsiekte sheep retrovirus envelope is receptor independent and does not require the surface domain. J Virol. 2003;77(11):6341–50. doi: 10.1128/JVI.77.11.6341-6350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis MC. Integrative pharmacology and drug discovery--is the tide finally turning? Nat Rev Drug Discov. 2006;5(5):377–9. doi: 10.1038/nrd2036. [DOI] [PubMed] [Google Scholar]
- De Las Heras M, Ortin A, Benito A, Summers C, Ferrer LM, Sharp JM. In-situ demonstration of mitogen-activated protein kinase Erk 1/2 signalling pathway in contagious respiratory tumours of sheep and goats. J Comp Pathol. 2006;135(1):1–10. doi: 10.1016/j.jcpa.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Digel W, Lubbert M. DNA methylation disturbances as novel therapeutic target in lung cancer: preclinical and clinical results. Crit Rev Oncol Hematol. 2005;55(1):1–11. doi: 10.1016/j.critrevonc.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Dutt A, Wong KK. Mouse models of lung cancer. Clin Cancer Res. 2006;12(14 Pt 2):4396s–4402s. doi: 10.1158/1078-0432.CCR-06-0414. [DOI] [PubMed] [Google Scholar]
- Fan H. Jaagsiekte sheep retrovirus and lung cancer. Springer-Verlag; Berlin: 2003. [Google Scholar]
- Girard L, Zochbauer-Muller S, Virmani AK, Gazdar AF, Minna JD. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res. 2000;60(17):4894–906. [PubMed] [Google Scholar]
- Hull S, Fan H. Mutational analysis of the cytoplasmic tail of jaagsiekte sheep retrovirus envelope protein. J Virol. 2006;80(16):8069–80. doi: 10.1128/JVI.00013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassim FA. PhD Thesis. University of Edinburgh; Edinburgh, UK: 1988. [Google Scholar]
- Jassim FA, Sharp JM, Marinello PD. Three-step procedure for isolation of epithelial cells from the lungs of sheep with jaagsiekte. Res Vet Sci. 1987;43(3):407–9. [PubMed] [Google Scholar]
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425(6956):407–10. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- Karni R, Mizrachi S, Reiss-Sklan E, Gazit A, Livnah O, Levitzki A. The pp60c-Src inhibitor PP1 is non-competitive against ATP. FEBS Lett. 2003;537(13):47–52. doi: 10.1016/s0014-5793(03)00069-3. [DOI] [PubMed] [Google Scholar]
- Khanna C, Lindblad-Toh K, Vail D, London C, Bergman P, Barber L, Breen M, Kitchell B, McNeil E, Modiano JF, Niemi S, Comstock KE, Ostrander E, Westmoreland S, Withrow S. The dog as a cancer model. Nat Biotechnol. 2006;24(9):1065–6. doi: 10.1038/nbt0906-1065b. [DOI] [PubMed] [Google Scholar]
- Kolch W. Ras/Raf signalling and emerging pharmacotherapeutic targets. Expert Opin Pharmacother. 2002;3(6):709–18. doi: 10.1517/14656566.3.6.709. [DOI] [PubMed] [Google Scholar]
- Liu SL, Lerman MI, Miller AD. Putative phosphatidylinositol 3-kinase (PI3K) binding motifs in ovine betaretrovirus Env proteins are not essential for rodent fibroblast transformation and PI3K/Akt activation. J Virol. 2003;77(14):7924–35. doi: 10.1128/JVI.77.14.7924-7935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Fu W, Ortin A, de las Heras M, Fan H. Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines. J Virol. 2005;79(7):4440–50. doi: 10.1128/JVI.79.7.4440-4450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Inoshima Y, Fruman DA, Brachmann SM, Fan H. Transformation of mouse fibrobalsts by jaagsiekte sheep retrovirus envelope does not require phosphatidylinositol 3-kinase. Journal of Virology. 2003;77:9951–9959. doi: 10.1128/JVI.77.18.9951-9959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Palmarini M, Murgia C, Fan H. Direct transformation of rodent fibroblasts by jaagsiekte sheep retrovirus DNA. Proc Natl Acad Sci U S A. 2001;98(8):4449–4454. doi: 10.1073/pnas.071547598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1(1):49–52. doi: 10.1016/s1535-6108(02)00027-2. [DOI] [PubMed] [Google Scholar]
- Mornex JF, Thivolet F, De las Heras M, Leroux C. Pathology of human bronchioloalveolar carcinoma and its relationship to the ovine disease. Curr Top Microbiol Immunol. 2003;275:225–48. doi: 10.1007/978-3-642-55638-8_9. [DOI] [PubMed] [Google Scholar]
- Palmarini M, Fan H. Retrovirus-induced ovine pulmonary adenocarcinoma, an animal model for lung cancer. J Natl Cancer Inst. 2001;93(21):1603–14. doi: 10.1093/jnci/93.21.1603. [DOI] [PubMed] [Google Scholar]
- Palmarini M, Fan H. Molecular biology of jaagsiekte sheep retrovirus. Curr Top Microbiol Immunol. 2003;275:81–115. doi: 10.1007/978-3-642-55638-8_4. [DOI] [PubMed] [Google Scholar]
- Palmarini M, Maeda N, Murgia C, De-Fraja C, Hofacre A, Fan H. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J Virol. 2001;75(22):11002–9. doi: 10.1128/JVI.75.22.11002-11009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmarini M, Sharp JM, De las Heras M, Fan H. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. Journal of Virology. 1999;73:6964–6972. doi: 10.1128/jvi.73.8.6964-6972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Rai SK, Duh FM, Vigdorovich V, Danilkovitch-Miagkova A, Lerman MI, Miller AD. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci U S A. 2001;98(8):4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ML, Carbone DP, Johnson DH. Improving the evaluation of new cancer treatments: challenges and opportunities. Nat Rev Cancer. 2003;3(4):303–9. doi: 10.1038/nrc1047. [DOI] [PubMed] [Google Scholar]
- Salvatori D, Gonzalez L, Dewar P, Cousens C, de las Heras M, Dalziel RG, Sharp JM. Successful induction of ovine pulmonary adenocarcinoma in lambs of different ages and detection of viraemia during the preclinical period. J Gen Virol. 2004;85(Pt 11):3319–24. doi: 10.1099/vir.0.80333-0. [DOI] [PubMed] [Google Scholar]
- Santarosa M, Favaro D, Quaia M, Galligioni E. Expression of heat shock protein 72 in renal cell carcinoma: possible role and prognostic implications in cancer patients. Eur J Cancer. 1997;33(6):873–7. doi: 10.1016/s0959-8049(97)00002-6. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000;97(20):10832–7. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju M, Sueoka N, Sato A, Iwanaga K, Sakao Y, Tomimitsu S, Tominaga M, Irie K, Hayashi S, Sueoka E. Hsp90 inhibitors cause G2/M arrest associated with the reduction of Cdc25C and Cdc2 in lung cancer cell lines. J Cancer Res Clin Oncol. 2006;132(3):150–8. doi: 10.1007/s00432-005-0047-7. [DOI] [PubMed] [Google Scholar]
- Sharp JM, Angus KW, Gray EW, Scott FM. Rapid transmission of sheep pulmonary adenomatosis (jaagsiekte) in young lambs. Brief report. Arch Virol. 1983;78(12):89–95. doi: 10.1007/BF01310861. [DOI] [PubMed] [Google Scholar]
- Suau F, Cottin V, Archer F, Croze S, Chastang J, Cordier G, Thivolet-Bejui F, Mornex JF, Leroux C. Telomerase activation in a model of lung adenocarcinoma. Eur Respir J. 2006;27(6):1175–82. doi: 10.1183/09031936.06.00125105. [DOI] [PubMed] [Google Scholar]
- Timpson P, Jones GE, Frame MC, Brunton VG. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr Biol. 2001;11(23):1836–46. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- Varela M, Chow YH, Sturkie C, Murcia P, Palmarini M. Association of RON tyrosine kinase with the Jaagsiekte sheep retrovirus envelope glycoprotein. Virology. 2006;350(2):347–357. doi: 10.1016/j.virol.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Varmus H, Pao W, Politi K, Podsypanina K, Du YC. Oncogenes come of age. Cold Spring Harb Symp Quant Biol. 2005;70:1–9. doi: 10.1101/sqb.2005.70.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wootton SK, Halbert CL, Miller AD. Sheep retrovirus structural protein induces lung tumours. Nature. 2005;434(7035):904–7. doi: 10.1038/nature03492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P. Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett. 2004;206(2):149–57. doi: 10.1016/j.canlet.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Yufu Y, Nishimura J, Nawata H. High constitutive expression of heat shock protein 90 alpha in human acute leukemia cells. Leuk Res. 1992;16(67):597–605. doi: 10.1016/0145-2126(92)90008-u. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kalyankrishna S, Wislez M, Thilaganathan N, Saigal B, Wei W, Ma L, Wistuba II, Johnson FM, Kurie JM. SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am J Pathol. 2007;170(1):366–76. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]