Abstract
Yersinia pestis is the etiologic agent of bubonic and pneumonic plagues. It is speculated that Y. pestis hijacks antigen-presenting cells (APCs), such as dendritic cells (DCs) and alveolar macrophages, in order to be delivered to lymph nodes. However, how APCs initially capture the bacterium remains uncharacterized. It is well known that HIV-1 uses human DC-specific intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN) (CD209) receptor, expressed by APCs, to be captured and delivered to target cell, such as CD4+ lymphocytes. Several gram-negative bacteria utilize their core lipopolysaccharides (LPS) as ligands to interact with the human DC-SIGN. Therefore, it is possible that Y. pestis, whose core LPS is naturally exposed, might exploit DC-SIGN to invade APCs. We demonstrate in this study that Y. pestis directly interacts with DC-SIGN and invades both DCs and alveolar macrophages. In contrast, when engineered to cover the core LPS, Y. pestis loses its ability to invade DCs, alveolar macrophages, and DC-SIGN-expressing transfectants. The interaction between Y. pestis and human DCs can be reduced by a combination treatment with anti-CD209 and anti-CD207 antibodies. This study shows that human DC-SIGN is a receptor for Y. pestis that promotes phagocytosis by DCs in vitro.
The genus Yersinia belongs to the gram-negative bacteria and is composed of 11 species (27, 57). Three Yersinia species are pathogenic to humans and animals. Yersinia pseudotuberculosis and Yersinia enterocolitica cause mild enteric diseases and are mainly transmitted by contaminated food and surface water. However, the etiologic agent of bubonic and pneumonic plagues is Yersinia pestis, the most virulent species. Y. pestis is also a young pathogen, directly evolved from Y. pseudotuberculosis within the last 10,000 to 20,000 years (1, 2).
All three pathogenic Yersinia species share a virulence plasmid, pCD1 (pYV), which is essential for pathogenic processes (8, 44). This plasmid encodes a type III secretion system, including LcrV (low-calcium response V, or V antigen) (16), YadA (a surface-expressed adherence molecule; however, yadA is a pseudogene in Y. pestis) (47, 51), and a number of cytotoxins and effectors that inhibit bacterial phagocytosis and processes of innate immunity (58). Y. pestis harbors two additional plasmids, pPCP1 (9.6 kb), which encodes the plasminogen activator (Pla), and pMT1 (pFra) (102 kb), which encodes the F1 capsule protein and a phospholipase D (Ymt). The products of these genes are necessary for tissue invasion (29) and infection of the plague flea vector (24, 50). Capsule formation by Y. pestis has been reported to confer antiphagocytic ability (18, 59). Recent studies further confirmed the important roles of pPCP1in the virulence of bubonic and pneumonic plagues (36, 50). However, despite extensive studies characterizing plasmid-encoded virulence determinants, the presence of pPCP1 and pMT1 does not account for the remarkable increase in virulence detected in Y. pestis. In fact, Y. pseudotuberculosis, even when transformed with additional virulence factors, such as the pPCP plasmid of Y. pestis, does not cause plague-like disease after subdermal injection (35).
Two other chromosomally located invasion genes, inv and ail, have been identified as important for the interaction of enteropathogenic Yersinia species with host cells (6, 25, 26, 40). Although Y. pestis does not express invasin, whose functions are probably replaced by a flea bite, Ail-like proteins have been reported (4, 34).
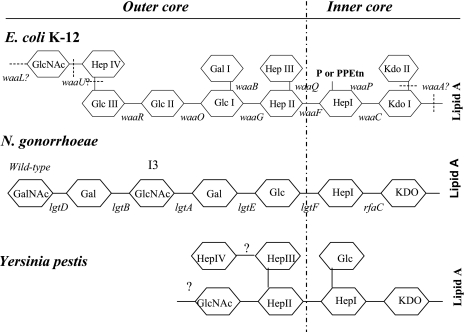
Another well-documented, but often neglected, virulence factor is the lipopolysaccharide (LPS), which plays a major role in the pathogenicity of gram-negative bacterial pathogens, such as Escherichia coli, Shigella, Klebsiella, Yersinia, and Salmonella, promoting toxicity and resistance to serum killing and phagocytosis (5, 10, 17, 41, 42, 48). LPS generally consists of three structural regions: (i) the lipid A backbone, (ii) an oligosaccharide core (core LPS), and (iii) the somatic O polysaccharide outer region (also called O antigen, O-specific antigen, or O-specific side chain) (Fig. 1). Core LPS is divided into inner and outer domains (Fig. 1). Strains of Y. pestis do not contain an O antigen (45), and therefore, the shortened LPS is also referred to as lipooligosaccharide (LOS). Gram-negative bacteria are classified as smooth or rough based on the presence or lack of the O antigen, respectively.
FIG. 1.
Structures of inner-core and outer-core regions of the LPS or LOS of Y. pestis, E. coli K-12, and N. gonorrhoeae and the genes involved in their synthesis. Genes involved in the biosynthesis of core LPS are shown at their approximate sites of action (solid lines). The sites that are variably substituted or still under investigation are indicated by dashed lines. GlcNAc, N-acetylglucosamine; GalNAc, N-acetylgalactosamine; Glc, glucose; Hep, heptose; Gal, galactose; P, phosphate; PPEtn, phosphoethanolamine; KDO, 2-keto-3-deoxyoctonate; PEA, phosphoethanolamine. It should be noted that Y. pestis, E. coli K-12, and N. gonorrhoeae do not naturally possess an O antigen.
Antigen-presenting cells (APCs) have been shown to be positioned to interact with Y. pestis immediately upon injection of the bacterium by an infected flea (58). It is generally accepted that APCs deliver Y. pestis to lymph nodes. Dendritic cells (DCs) are APCs and express a C-type lectin called DC-specific intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN) (CD209), an innate immune receptor (56) that can interact with several bacterial species (55, 56). DC-SIGN is also expressed in certain types of macrophages (23), including human alveolar macrophages (54). This model is reminiscent of how human immunodeficiency virus type 1 (HIV-1) targets APCs. It is well established that HIV-1 hijacks DC-SIGN so as to be captured and trafficked to target cells, such as CD4 lymphocytes (19, 21, 39).
Recently, we showed that DC-SIGN is a receptor for the core LPSs of several gram-negative bacterial strains, promoting bacterial adherence and phagocytosis (32, 61, 62). In this study, we explore the model that (i) after Y. pestis overcomes the first line of host defense, such as the skin via a flea bite, it encounters secondary host defense systems, such as macrophages and DCs (Langerhans cells in the skin) (38) or alveolar macrophages through aspiration, and (ii) APCs capture Y. pestis through a core LPS-DC-SIGN interaction.
MATERIALS AND METHODS
Bacterial strains. (i) E. coli.
E. coli strain K-12 CS180 contains core LPS but lacks O antigen (49). CS1861 is the strain of CS180 harboring pSS37, a plasmid containing all of the genes necessary for the expression of the Shigella dysenteriae 1 O antigen (31, 33, 49) (Fig. 1). E. coli strains were cultured on Luria-Bertani medium (LB) supplemented with 1.5% agar at 37°C overnight.
(ii) Y. pseudotuberculosis.
Y. pseudotuberculosis Y1 is a serotype O:1a strain lacking the virulence plasmid (pYV) and expression of the Ail protein (unpublished data). The strain was from CDC and was once used as a control strain for invasion (11).
(iii) Y. pestis.
All strains of Y. pestis used in this study are derived from the KIM strain (20). The O antigen-expressing KIM-6/pAY100.1 was constructed by transformation of the pAY100.1 plasmid from strain KIM-D27/pAY100.1, which expresses the O antigen from Y. enterocolitica serotype O:3 (43). The strains were cultured on gonococcus (GC)-based plates (Difco, Sparks, MD) supplemented with 1% hemoglobin (USB Co., Cleveland, OH).
Due to concerns about biosafety, all Yersinia strains used in this study were virulence plasmid-cured and/or Pgm-negative strains, selected using a combination of magnesium oxalate and Congo red selection methods (46).
(iv) N. gonorrhoeae.
Neisseria gonorrhoeae strain F62 was cultured on GC-based-plates (Difco, Sparks, MD) supplemented with 1% IsovitaleX and maintained as previously described (12, 14). N. gonorrhoeae is the etiologic agent for gonorrhea, a sexually transmitted disease. An isogenic mutant, I3, derived from wild-type F62, contains a mutation within lgtB (lgtB mutant) and binds to the DC-SIGN receptor (61, 62). This mutant was a gift from E. C. Gotschlich at Rockefeller University (Fig. 1) (22). For N. gonorrhoeae, only Opa− (opacity protein-negative) and pilus− variants were used.
Cellular-biology reagents.
Anti-CD209 and -CD207 monoclonal antibodies specific for human DC-SIGN and human langerin, respectively, were purchased from Pharmingen (San Diego, CA) and R&D Systems (Minneapolis, MN). YTH71.3, an antibody that recognizes CEACAM1 (CD66a), CEACAM6 (CD66c), and CEACAM3 (CD66d), was purchased from Roche (Indianapolis, IN). Anti-Y. pestis polyclonal antiserum was generated from rabbits challenged with KIM-6 cultured at 21°C (28). Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated anti-rabbit immunoglobulin G (whole-molecule) antibodies produced in goats were purchased from Sigma (St. Louis, MO). Mouse anti-F antigen monoclonal antibody and the anti-mouse antibodies conjugated with FITC and PE were purchased from eBiomedical Sciences (San Diego, CA).
Mannan, the ligand antagonist of human mannose receptor, was purchased from Sigma (St. Louis, MO).
Mermaid is a DC-SIGN-like molecule expressed by the marine nematode Laxus oneistus. The carbohydrate recognition domain of Mermaid shares both structural and functional similarity with that of DC-SIGN as described previously (9). A recombinant form of Mermaid (His-Mermaid) was expressed and purified as described previously (9).
Preparation of human DCs.
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats obtained from human blood donors by density gradient centrifugation over Ficoll-Paqueplus (1.077 g/ml; Pharmacia, Piscataway, NJ). Blood was loaded in a 1:1 (vol/vol) ratio on Ficoll and centrifuged without braking for 30 min. The PBMC were washed four times with phosphate-buffered saline (PBS), and monocytes were purified from the PBMC using CD14 microbeads (Miltenyi Biotec, Auburn, CA) as previously described (37). To increase purity, the cells were passed over a second CD14 microbead column. The final purity of the isolated monocytes was >98% as assessed by labeling with CD14-FITC antibody (Caltag, Carlsbad, CA) and flow cytometric analysis. Purified CD14-positive (CD14+) monocytes (5 × 105 cells/ml) were cultured for 6 days to promote differentiation of immature monocyte-derived DCs in culture medium consisting of RPMI 1640 (BioWhittaker, Walkersville, MD), 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 100 U/ml penicillin, and 100 μg/ml streptomycin in the presence of 20 ng/ml recombinant human granulocyte-macrophage colony-stimulating factor (Immunex, Seattle, WA) and 10 ng/ml recombinant human interleukin 4 (Peprotech, Rock Hill, NJ). The DCs derived from these cultured monocytes display typical dendrites, and in mixed lymphocyte cultures, they promote activation of alloreactive T cells. The phenotype of these cells is HLA-DR+ CD1a+ CD86+ CD40+ CD14− (37). Upon LPS stimulation, these DCs express CD83 (63, 64). We have utilized this protocol for identification of DC-SIGN as a receptor for the core LPSs of several gram-negative bacteria and to explore how N. gonorrhoeae enhances HIV infection (32, 60, 61).
Human alveolar macrophage isolation and treatment.
Bronchoalveolar lavage (BAL) cells were collected from volunteers following a protocol described recently by Tailleux et al. (54). Acquisition of the BAL cells was approved by the Institutional Review Board and Study Committees at the College of Medicine—Rockford, University of Illinois. The collected BAL cells were washed and cultured in RPMI 1640 (BioWhittaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal calf serum (FCS) (HyClone, Logan, UT), 2 mM l-glutamine, and antibiotics in a 96-well plate for 3 h at 37°C before nonadherent cells were removed. Half of the cells were treated with interleukin 4 (10 ng/ml; Peprotech, Rock Hill, NJ), tumor necrosis factor alpha (50 ng/ml; Peprotech, Rock Hill, NJ), and E. coli-derived LPS (100 ng/ml; Sigma, St. Louis, MO). Invasion experiments followed, after the two sets of cells were placed at 37°C with 5% CO2 for 2 days.
Cultured cell lines.
HeLa-DC-SIGN cells were generated by transfecting HeLa cells with human DC-SIGN cDNA, followed by selection for stable surface DC-SIGN expression as originally described (52). The cell lines were recently used for identification of core LPSs from several gram-negative bacteria as ligands for the DC-SIGN receptor (32, 61, 62).
Adherence and phagocytosis assays.
The assays for adherence and phagocytosis have been described previously (12, 14). Briefly, host cells (HeLa cells, DCs, and alveolar macrophages) were plated in 24- or 96-well plates. The cells were suspended in RPMI with 2% FCS at a concentration of 4 × 105/ml. One-half milliliter each of these cell suspensions was added to 24-well plates, and after the addition of 50 μl of bacterial suspensions at a concentration of 1 × 107 CFU/ml, the cells were allowed to incubate for 2.5 h (2 h for DCs and alveolar macrophages) at 37°C in the presence of 5% CO2. The cell monolayers were then washed three times with PBS. The number of associated bacteria (adherent and internalized) per cell was quantified by washing the cells three times with RPMI containing 2% FCS and plating the culture after the cells were lysed by 0.5% saponin (Calbiochem Corp., San Diego, CA).
To determine the internalization of bacteria, gentamicin, which kills extracellular bacteria but cannot penetrate into host cells, was added to each well to a final concentration of 100 μg/ml, and the cultures were incubated for 60 min. The cells were washed twice to remove the antibiotics (for DCs, cytospin is necessary during the washing, since DCs do not attach to plastic wells). Then, the cells were suspended in PBS containing 0.5% saponin, diluted, and plated on GC or LB plates. The level of internalization of bacteria in these host cells was calculated by determining the number of CFU recovered from lysed cells. All experiments were performed in triplicate, and data were expressed as means ± standard errors. Statistical significance was calculated using Student's t test.
For the inhibition assay, reagents were added 20 min prior to the addition of bacteria at the following concentrations: anti-DC-SIGN antibody, 5 μg/ml; DC-SIGN-like protein (Mermaid), 10 μg/ml; and mannan, 500 μg/ml. The concentrations used were based on our preliminary data and were selected based on the fact that at these concentrations, there was no influence on the survival of bacteria and HeLa cells or the interaction between pEXI and HeLa-CEACAM3 (12, 13), as recently shown (32, 61, 62).
Statistical analysis.
Statistical significance was mainly calculated using Statview, a statistical program from SAS Institute, North Carolina. Because all statistical analyses described were single comparisons, only Student's t test was utilized.
Determination of phagocytosis by flow cytometry.
The following method was used to supplement the survival-based phagocytosis assay described above (61). The measurement of the phagocytosis rate of bacteria by DCs was adapted as follows. Briefly, bacteria were suspended in RPMI medium containing 5- and 6-carboxyfluorescein diacetate, succinimidyl ester (Molecular Probes, Eugene, OR), for 40 min and washed twice with RPMI to remove the excess dye. Labeled bacteria were added to the DC cultures for 2 h. The cell cultures were washed twice to remove unbound bacteria. The DCs plus associated bacteria were fixed with 2% paraformaldehyde. Before flow cytometry, a 1:10 dilution of trypan blue (0.4%; Sigma, St. Louis, MO) was added to the fixed cell cultures, and the mixture was incubated at ambient temperature for 10 min (61) to quench the fluorescence from extracellularly labeled bacteria. Trypan blue blocks fluorescence but cannot penetrate host cells; therefore, fluorescence from internalized bacteria is not influenced by the addition of trypan blue. The rate of bacterial internalization was determined by comparing the percentage of fluorescence-positive DCs with that of unlabeled DCs.
Microscopic assay to determine cellular interactions.
Purified DCs and HeLa-DC-SIGN cells were plated in 24-well plates, with each well containing a coverslip. The host cells were infected with Y. pestis following the same procedures described for the interaction assays. After a 2-h incubation, Y. pestis-infected DCs and HeLa-DC-SIGN cells were washed twice with PBS with cytospin and fixed with 2% paraformaldehyde in PBS. Infected DCs on the coverslips were stained with anti-Y. pestis antiserum from rabbits for 45 min. Subsequently, FITC-anti-rabbit antibody (the second antibody) was used to label the extracellular bacteria (green). The coverslips were treated with PBS containing 0.5% Triton X-100 for 10 min to make holes in the membranes of the DCs and HeLa-DC-SIGN cells. After the cells were washed three times with PBS, the same anti-Y. pestis antiserum was added for 45 min to stain the intracellular Y. pestis cells. Following three washes, the PE-conjugated anti-rabbit antibody was added to label the intracellular bacteria (red). The numbers of adherent (green) and internalized (red) bacteria per host cell were determined by using a fluorescence microscope and counting the bacteria associated with 100 host cells on coverslips. It should be noted that Y. pestis cultured at 37°C did not stain well with this rabbit antiserum, and therefore, the anti-F antigen monoclonal antibody was used instead of anti-Y. pestis polyclonal antiserum. Corresponding anti-mouse immunoglobulin G secondary antibodies conjugated with FITC and PE were used.
RESULTS
Y. pestis cultured at 26°C or 6°C, but not 37°C, invades and survives inside DCs.
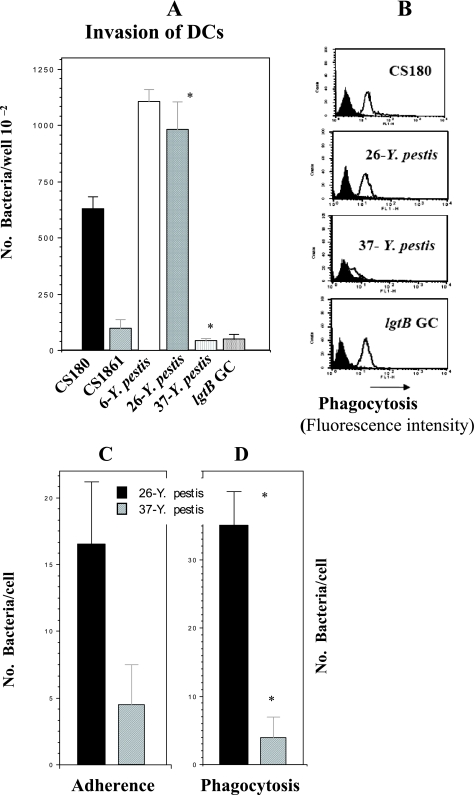
We examined the ability of Y. pestis KIM-6 cultured at 37°C, 26°C, and 6°C to invade DCs (Y. pestis is capable of replication at 6°C, although the generation time is significantly increased). (Y. pestis cells cultured at 37°C, 26°C, and 6°C are referred to as 37-Y. pestis, 26-Y. pestis, and 6-Y. pestis, respectively, hereafter.) We used E. coli strains K-12 CS180 (a strain with the core LPS exposed) and CS1861 (CS180 expressing an O antigen) as controls. We have used this pair of strains to demonstrate that exposure of the core LPS of E. coli is essential to initiate core LPS-DC interaction (32, 61). The N. gonorrhoeae F62 lgtB mutant was also used as a control. We and others have recently shown that the Neisseria lgtB mutant invades DCs (53, 61).
The results from both a gentamicin protection assay (Fig. 2A) and flow cytometry (Fig. 2B) show that 6- and 26-Y. pestis, but not 37-Y. pestis, invades DCs. 37-Y. pestis expresses the F1 capsule, which most likely blocks or inhibits the interaction between the potential receptors and Y. pestis. These results were also confirmed by microscopic approaches (Fig. 2C and D). A spontaneous and undefined F1− mutant of KIM5 recovered its ability to invade DCs at all temperatures, supporting this conclusion (data not shown). An important issue that should be recognized is that the gentamicin killing assay is dependent on bacterial survival inside host cells. As the flow cytometric data show, Y. pestis, E. coli, and N. gonorrhoeae promote similar levels of internalization, but Y. pestis was recovered at a higher rate than E. coli, suggesting that Y. pestis survives inside DCs very well. In contrast, the recovery rate for N. gonorrhoeae was very low, similar to the previously described results (61).
FIG. 2.
26- or 6-Y. pestis invades DCs. Gentamicin protection-, flow cytometry-, and fluorescence microscopy-based assays were used to determine the DC invasion rates of Y. pestis. E. coli strain K-12 (CS180 and CS1681) and the N. gonorrhoeae F62 lgtB mutant were utilized as controls, whose invasive nature has been reported recently (32, 61, 62). (A) Data from the gentamicin protection assay. (B) Data from flow cytometry. Labeled and unlabeled bacteria are shown in open and filled curves, respectively. (C and D) Adherence (C) and phagocytosis (D) data acquired from fluorescence microscopy assays. (A and D) *, P < 0.001, calculated by Student's t test, in the comparison of the invasion of DCs by 26- and 37-Y. pestis. n was nine samples for each test. The error bars indicate standard errors.
Temperature regulates the interaction of Y. pestis with DC-SIGN.
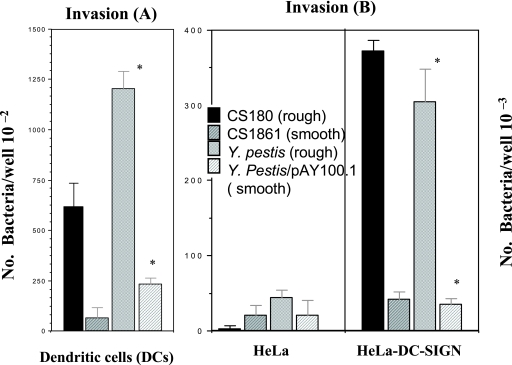
The DC-SIGN receptor on DCs is responsible for interaction with the core LPSs of several gram-negative bacteria (32, 61, 62). To investigate if DC-SIGN is responsible for the interaction of DCs with Y. pestis, a stably transfected DC-SIGN HeLa cell line (HeLa-DC-SIGN) was tested for its ability to phagocytose 26- and 6-Y. pestis. Again, E. coli CS180 and CS1861 were used as controls for core LPS involvement. Y. pseudotuberculosis was also used as a control, since it may express three invasion factors—invasin, YadA, and Ail—and invade most epithelial cells (6, 25, 40).
26- and 6-Y. pestis promotes a typical and strong DC-SIGN-mediated invasion reaction (Fig. 3B and C). There is no interaction of HeLa and HeLa-DC-SIGN cells with 37-Y. pestis, probably due to F1 capsule formation. Finally, Fig. 3 also shows that 26- and 6-Y. pestis can interact with HeLa cells (Fig. 3A), which is independent from the interaction with DC-SIGN. We address this phenomenon below and in the Discussion. In conclusion, Y. pestis interacts with the DC-SIGN receptor directly.
FIG. 3.
HeLa-DC-SIGN cells phagocytose 26-Y. pestis. (A and B) The phagocytoses of two sets of bacteria, E. coli K-12 (CS180 and CS1861) and 6-, 26-, and 37-Y. pestis, with HeLa (A) and HeLa-DC-SIGN cells (B) were performed by incubating the cell lines for 2.5 h with the indicated bacterial strains and by killing the extracellular bacteria with 100 μg/ml (final concentration) of gentamicin as described in Materials and Methods. The number of phagocytosed bacteria was determined by counting CFU recovered following gentamicin treatment. (C) Results from fluorescence microscopy assay. Only data for Y. pestis are shown. *, P < 0.001, based on Student's t test comparing the interaction of HeLa-DC-SIGN cells with 26-Y. pestis to the interaction of HeLa with the same bacterium. n was nine samples for each test. The error bars indicate standard errors.
Inhibition of the Y. pestis-host cell interaction by heparin, anti-DC-SIGN antibody, mannan, and a DC-SIGN-like molecule.
Figure 3 shows that 26- and 6-Y. pestis can also interact with HeLa cells, which is independent from the interaction with DC-SIGN. Our preliminary data showed that this DC-SIGN-independent interaction is in part mediated by cell surface heparan sulfate proteoglycan receptor, which can be inhibited by the addition of heparin, a synthetic form of heparan sulfate (Fig. 4A). We present this information because heparin was added in the remaining assays measuring bacterium-HeLa-DC-SIGN cell interaction in order to eliminate or reduce some non-DC-SIGN-specific interaction between Y. pestis and epithelial cells.
FIG. 4.
DC-SIGN-mediated phagocytosis of Y. pestis is inhibited by heparin, anti-DC-SIGN antibody, mannan, and DC-SIGN-like Mer- maid. (A) E. coli K-12 CS180, Y. pseudotuberculosis serotype O:1a, and 26-Y. pestis were incubated with HeLa and HeLa-DC-SIGN cells for 2.5 h in the presence or absence of heparin (30 μg/ml). (B) 26-Y. pestis was incubated with HeLa-DC-SIGN cells for 2.5 h in the presence or absence of anti-CD66 (5 μg/ml) and DC-SIGN (5 μg/ml), mannan (500 μg/ml), and DC-SIGN-like protein (His-Mermaid; 10 μg/ml). All reagents were added to the media for 20 min before the addition of bacteria. Heparin (30 μg/ml) was added to every sample in order to eliminate the non-DC-SIGN-specific interaction. The concentration of each reagent used in the experiment was based on previously published data (32, 61, 62). The phagocytosis rate of Y. pestis was evaluated by the recovery of bacteria from gentamicin protection. E. coli K-12 CS180 and Y. pseudotuberculosis serotype O:1a were control strains. In addition, only half the number of Y. pseudotuberculosis cells were added to the HeLa-DC-SIGN sample in order to better compare the effects of these reagents among the three strains. (A) Y. pseudotuberculosis promotes effective internalization into HeLa cells. *, P < 0.001, based on Student's t test comparing the interaction of HeLa-DC-SIGN cells with 26-Y. pestis in the presence of anti-DC-SIGN antibody to the interaction without the presence of antibody. n was nine samples for each test. The error bars indicate standard errors.
To verify that the interaction of Y. pestis with DC-SIGN was specific, we examined whether core LPS-DC-SIGN could be inhibited by anti-DC-SIGN antibody; mannan, which specifically binds mannose-related receptors; and a recombinant form of Mermaid (His-Mermaid), a newly identified DC-SIGN-like protein. The carbohydrate recognition domain of Mermaid shares both structural and functional similarity with that of DC-SIGN (9); therefore, a recombinant form of Mermaid was able to inhibit the core LPS-DC-SIGN interaction (62). Anti-CD66 antibody was employed as a control antibody. In addition, E. coli K-12 CS180 and Y. pseudotuberculosis serotype O:1a grown at 26°C, mediating a DC-SIGN-dependent and -independent interaction, respectively, were utilized as control strains. Anti-DC-SIGN antibody and His-Mermaid inhibited invasion of 26-Y. pestis in HeLa-DC-SIGN cells (Fig. 4B). Further, mannan demonstrated the strongest inhibition ability. It should be noted that the concentrations of anti-DC-SIGN antibody, mannan, Mermaid, and heparin used in this study did not influence the viability of either bacteria or HeLa cells (62). Together, the data indicate that there is a specific interaction between DC-SIGN and LPS/LOS of Y. pestis.
Reduction of DC phagocytosis of Y. pestis by combination of anti-DC-SIGN and -langerin (CD207) antibodies.
As indicated in our recent publications, the anti-DC-SIGN antibody was not effective in inhibition of the LPS core-mediated phagocytosis of E. coli and N. gonorrhoeae by DCs (32, 61, 62). We have speculated that, besides DC-SIGN, other receptors might also be involved in the phagocytosis of the core-exposed gram-negative bacteria. Fortunately, our recent unpublished observations indicate that langerin (CD207) from APCs is also a receptor for Y. pestis. Therefore, the experiments shown in Fig. 2 were performed in the presence of these two antibodies. As shown in Fig. 5, both antibodies, if used separately, failed to show any significant impact on the phagocytosis of Y. pestis. However, when the two antibodies were added together, the phagocytosis of Y. pestis by DCs was indeed reduced. Nevertheless, this reduction was not as strong as the inhibition of the interaction of 26-Y. pestis with HeLa-DC-SIGN cells (Fig. 4), suggesting that additional receptors for Y. pestis are present on DCs. We are currently investigating this observation further.
FIG. 5.
Reduction of phagocytosis of Y. pestis by DCs in the presence of anti-DC-SIGN and -langerin (CD207) antibodies. Other than the addition of the anti-CD209 and anti-CD207 antibodies, the same procedures were followed as for DCs in Fig. 2. *, P < 0.001 by Student's t test comparing the interaction of DCs with 26-Y. pestis in the presence of anti-DC-SIGN and CD207 antibodies to the interaction without the presence of antibody. n was nine samples for each test. The error bars indicate standard errors.
O antigen-expressing Y. pestis loses the ability to interact with DCs and DC-SIGN.
Our previous publications (32, 61, 62) and the inhibition assays (Fig. 4) clearly suggested that interaction of Y. pestis with DCs and DC-SIGN is due to the exposure of core LPS/LOS. However, it is possible that other surface components might bind to DC-SIGN when O antigen is deleted during evolution. Y. pestis might utilize other surface components, rather than core LPS, to interact with DCs and DC-SIGN. We used the KIM-6/pAY100.1 strain (smooth), which expresses O antigen from Y. enterocolitica serotype O:3 (43), to address this concern. We hypothesized that the ability of 26-Y. pestis to interact with DCs and DC-SIGN would be inhibited when the exposed core LPS is shielded by the expression of O antigen. Figure 6 shows that DCs (Fig. 6A) and HeLa-DC-SIGN cells (Fig. 6B) lost the ability to promote effective phagocytosis of Y. pestis expressing O antigen (KIM-6/pAY100.1). As indicated above, the interaction assay with HeLa-DC-SIGN cells was performed in the presence of heparin.
FIG. 6.
O antigen-expressing Y. pestis loses the ability to invade DCs and HeLa-DC-SIGN cells. Y. pestis KIM (rough)/Y. pestis KIM-6/pAY100.1 (smooth) were tested for the ability to invade DCs (A) and HeLa-DC-SIGN cells (B). E. coli K-12 CS1861 (smooth)/CS180 (rough) were used as controls. The same methods described in the legends to Fig. 2 and 3 were applied in these experiments. *, P < 0.001 by Student's t test comparing the interaction of DCs (A) and HeLa-DC-SIGN cells (B) with Y. pestis KIM-6 (rough) to interaction with Y. pestis KIM-6/pAY100.1 (smooth). n was nine samples for each test. The error bars indicate standard errors.
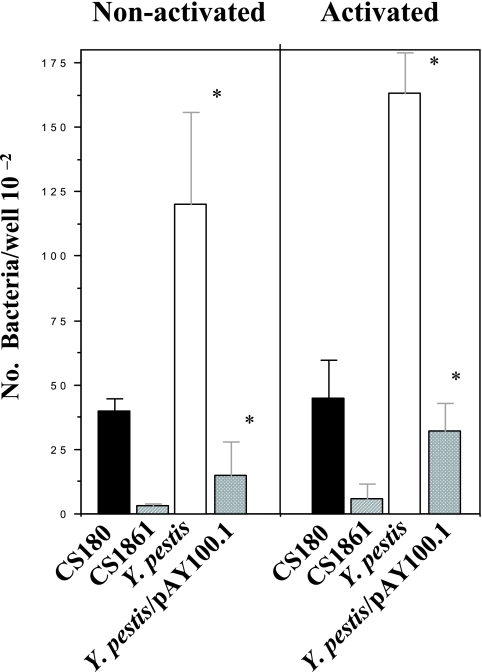
Y. pestis, but not its O antigen-expressing derivative, invades human alveolar macrophages.
The approaches described above were designed to address the initial stages of bubonic rather than pneumonic plague. With regard to biodefense concerns, pneumonic plague is considerably more worrisome, as the disease could initiate widespread pathogen dissemination. Therefore, we sought to determine which host immune cells are the initial targeting cells for pneumonic plague. DC-SIGN is synthesized on certain types of macrophages (23), especially human alveolar macrophages (54). We subsequently studied the role human alveolar macrophages play in Y. pestis infection using the methods described for human DCs. Again, two sets of strains were used for this study: Y. pestis KIM-6 (rough)/KIM-6/pAY100.1 (smooth) and E. coli K-12 CS180 (rough)/CS1861 (smooth). We also explored whether there were any differences between the phagocytosis of these bacteria and activated/nonactivated macrophages. Figure 7 shows that Y. pestis KIM-6 (rough) and E. coli K-12 CS180 (rough), rather than their smooth counterparts, invade human alveolar macrophages. It also appears that activated alveolar macrophages phagocytose more bacteria (Fig. 7B) than nonactivated macrophages, but without any statistical significance. All strains used were grown at 26°C, and the experiments were performed in the absence of heparin. This result suggests that phagocytosis of these bacteria by human alveolar macrophages is also via core LPS-DC-SIGN interaction. In short, Y. pestis, but not its O antigen-expressing derivative, is able to invade and survive effectively in human alveolar macrophages.
FIG. 7.
Interaction of human alveolar macrophages with Y. pestis. Purified human alveolar macrophages were plated on 96-well plates. Half of the plated cells were activated by the addition of activators (B). The invasion assay followed the same procedures as for DCs in Fig. 2. The two pairs of strains used were Y. pestis KIM-6/Y. pestis KIM-6/pAY100.1 and E. coli K-12 CS180/E. coli K12 CS1861. *, P < 0.001 by Student's t test comparing the interaction of alveolar macrophages with Y. pestis KIM-6 (rough) to interaction with Y. pestis KIM-6/pAY100.1 (smooth). n was nine samples for each test. The error bars indicate standard errors.
DISCUSSION
Recently, we observed that the LOS/LPS core saccharides of several gram-negative pathogens act as ligands for DC-SIGN, facilitating phagocytosis by DCs (32, 61, 62). However, it is well known that the O antigen plays a major role in the pathogenicity of gram-negative bacterial pathogens, such as E. coli, Shigella, Klebsiella, and Salmonella, promoting resistance to serum killing and phagocytosis (5, 10, 17, 42).
An important corollary to this hypothesis is that some pathogens, and probably Y. pestis, have evolved mechanisms for exploiting the very host defenses designed to eliminate them, resulting in an expanded ability to disseminate. Given that Y. pestis cannot produce an O antigen, we suggest that the exposure of the Y. pestis core LOS/LPS to DCs plays a fundamental role in the pathogenic process. Our results support the hypothesis that DCs use the DC-SIGN receptor to capture Y. pestis and may deliver the bacterium to lymph nodes to establish infection.
The idea that DCs use DC-SIGN to capture microbial pathogens for delivery to lymphocytes has been emerging since the discovery of DC-SIGN as a receptor for the gp120 antigen of HIV-1. Extensive studies of the relationship of HIV-DC-SIGN have established that DCs serve as the carrier for HIV-1, with DC-SIGN as the receptor for viral particles and delivering them to target cells, such as CD4 lymphocytes (21, 39). This concept appears to apply to Y. pestis, as well.
Y. pestis must be resistant to killing and digestion by DCs. Other investigators have reported that Y. pestis survives in macrophages (7, 15, 18). The phagocytosis experiments we used in this study are based on the ability for bacterial survival inside host cells, because gentamicin kills the extracellular bacteria but does not penetrate host cells. We were amazed to observe that Y. pestis survives inside DCs extremely well compared to N. gonorrhoeae (Fig. 2) or E. coli. Indeed, Y. pestis possessed the strongest ability to survive inside DCs among the bacterial species (E. coli, Salmonella, Haemophilus, and Neisseria) we have tested to date (unpublished data).
Although Y. pestis does not express invasin, it promotes a DC-SIGN-independent interaction with HeLa cells (Fig. 3 and 4). However, the interaction was inhibited by the addition of heparin, a synthetic form of heparan sulfate (Fig. 4A). Our unpublished data indicate that Ail-mediated interaction with epithelial cells involves the heparan sulfate proteoglycan. Therefore, to reduce the background (DC-SIGN-independent interaction), we added heparin in some Y. pestis interaction experiments.
In summary, we believe that this study has suggested four significant points. (i) The study indicates a concept for Y. pestis infection; after overcoming the initial, nonspecific immune defense system (e.g., the skin), Y. pestis may induce DCs, through the core LPS-DC-SIGN interaction, to be captured and carried to lymph nodes, utilizing a mechanism similar to that demonstrated in HIV-DC-SIGN interaction. (ii) Expression of O antigen usually enhances bacterial biological effects. However, our studies indicate that the loss of O antigen during evolution may be an important trait necessary for Y. pestis to disseminate from the flea bite site and establish infection at the lymph node. Y. pestis strains have evolved mechanisms for exploiting the very host defenses designed to eliminate them, in order to disseminate to other parts of the host. (iii) The study will help us better understand how the life cycle of Y. pestis impacts infection. Given its unique life cycle, Y. pestis must survive and grow in a broad range of temperatures from 4°C to 41°C (7). During hibernation of rodents in winter, the temperature of the fleas associated with these animals is about 5°C (30). In the other seasons, fleas reside in rodent burrows or mammalian hair with temperatures fluctuating between 21°C and 28°C. After establishment of infection, Y. pestis survives and grows within bodies and body fluids of mammals (37°C to 41°C) (3). As shown in our data, Y. pestis grown at 6°C and 26°C, rather than 37°C, interacts with DCs and DC-SIGN. This may explain how Y. pestis from fleas is infectious to humans. Once delivered to lymph nodes, Y. pestis needs to overcome host immune systems by expressing factors, such as capsule, at 37°C to attack host cells. (iv) We emphasize that all the strains used in this work did not carry the virulence plasmid and were therefore not able to inhibit endocytosis by host cells, as the normal wild-type strain does. Therefore, we suggest that binding to DCs while blocking endocytosis via an active type III secretion system may readily allow the transport of viable bacteria to lymph nodes. (v) Human DC-SIGN is a cellular receptor for Y. pestis.
Acknowledgments
We thank Nawal Lutfiyya at College of Medicine—Rockford, University of Illinois, for her help with statistical analysis. We thank Ines Chen and Emil C. Gotschlich for useful suggestions and editorial comments on the manuscript. We are indebted to Emil C. Gotschlich for his continual collegiality with regard to the supply of Neisseria strains.
This work was supported by PHS grants and an internal grant from the College of Medicine—Rockford, University of Illinois, to T. Chen. The work in the Skurnik laboratory is supported by funding from the Academy of Finland (grant 114075).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 19 February 2008.
REFERENCES
- 1.Achtman, M., G. Morelli, P. Zhu, T. Wirth, I. Diehl, B. Kusecek, A. J. Vogler, D. M. Wagner, C. J. Allender, W. R. Easterday, V. Chenal-Francisque, P. Worsham, N. R. Thomson, J. Parkhill, L. E. Lindler, E. Carniel, and P. Keim. 2004. Microevolution and history of the plague bacillus, Yersinia pestis. Proc. Natl. Acad. Sci. USA 10117837-17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 9614043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisimov, A. P., L. E. Lindler, and G. B. Pier. 2004. Intraspecific diversity of Yersinia pestis. Clin. Microbiol. Rev. 17434-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartra, S. S., K. L. Styer, D. M. O'Bryant, M. L. Nilles, B. J. Hinnebusch, A. Aballay, and G. V. Plano. 2007. Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein. Infect. Immun. 76612-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengoechea, J. A., H. Najdenski, and M. Skurnik. 2004. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 52451-469. [DOI] [PubMed] [Google Scholar]
- 6.Bliska, J. B., M. C. Copass, and S. Falkow. 1993. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect. Immun. 613914-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brubaker, R. R. 1991. Factors promoting acute and chronic diseases caused by yersiniae. Clin. Microbiol. Rev. 4309-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker, R. R. 1983. The Vwa+ virulence factor of yersiniae: the molecular basis of the attendant nutritional requirement for Ca++. Rev. Infect. Dis. 5(Suppl. 4)S748-S758. [DOI] [PubMed] [Google Scholar]
- 9.Bulgheresi, S., I. Schabussova, T. Chen, N. P. Mullin, R. M. Maizels, and J. A. Ott. 2006. A new C-type lectin similar to the human immunoreceptor DC-SIGN mediates symbiont acquisition by a marine nematode. Appl. Environ. Microbiol. 722950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns, S. M., and S. I. Hull. 1998. Comparison of loss of serum resistance by defined lipopolysaccharide mutants and an acapsular mutant of uropathogenic Escherichia coli O75:K5. Infect. Immun. 664244-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, T., R. Belland, J. Wilson, and J. Swanson. 1995. Adherence of pilus− Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J. Exp. Med. 182511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, T., S. Bolland, I. Chen, J. Parker, M. Pantelic, F. Grunert, and W. Zimmermann. 2001. The CGM1a (CEACAM3/CD66d) mediated phagocytic pathway of Neisseria gonorrhoeae expressing Opacity (Opa) proteins is also the pathway to cell death. J. Biol. Chem. 27617413-17419. [DOI] [PubMed] [Google Scholar]
- 13.Chen, T., and E. Gotschlich. 1996. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc. Natl. Acad. Sci. USA 9314851-14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, T., F. Grunert, A. Medina-Marino, and E. Gotschlich. 1997. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med. 1851557-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23861-867. [DOI] [PubMed] [Google Scholar]
- 17.Cortes, G., N. Borrell, B. de Astorza, C. Gomez, J. Sauleda, and S. Alberti. 2002. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect. Immun. 702583-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 701453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engering, A., S. J. Van Vliet, T. B. Geijtenbeek, and Y. Van Kooyk. 2002. Subset of DC-SIGN+ dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood 1001780-1786. [DOI] [PubMed] [Google Scholar]
- 20.Fetherston, J. D., P. Schuetze, and R. D. Perry. 1992. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol. Microbiol. 62693-2704. [DOI] [PubMed] [Google Scholar]
- 21.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100587-597. [DOI] [PubMed] [Google Scholar]
- 22.Gotschlich, E. 1994. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J. Exp. Med. 1802181-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granelli-Piperno, A., A. Pritsker, M. Pack, I. Shimeliovich, J. F. Arrighi, C. G. Park, C. Trumpfheller, V. Piguet, T. M. Moran, and R. M. Steinman. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 1754265-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinnebusch, B. J., A. E. Rudolph, P. Cherepanov, J. E. Dixon, T. G. Schwan, and A. Forsberg. 2002. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 296733-735. [DOI] [PubMed] [Google Scholar]
- 25.Isberg, R., L. Deborah, S. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50769-778. [DOI] [PubMed] [Google Scholar]
- 26.Isberg, R., and S. Falkow. 1985. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature 317262-264. [DOI] [PubMed] [Google Scholar]
- 27.Isberg, R. R., and P. Barnes. 2001. Subversion of integrins by enteropathogenic Yersinia. J. Cell Sci. 11421-28. [DOI] [PubMed] [Google Scholar]
- 28.Jarrett, C. O., E. Deak, K. E. Isherwood, P. C. Oyston, E. R. Fischer, A. R. Whitney, S. D. Kobayashi, F. R. DeLeo, and B. J. Hinnebusch. 2004. Transmission of Yersinia pestis from an infectious biofilm in the flea vector. J. Infect. Dis. 190783-792. [DOI] [PubMed] [Google Scholar]
- 29.Karlyshev, A. V., E. E. Galyov, O. Smirnov, A. P. Guzayev, V. M. Abramov, and V. P. Zav'yalov. 1992. A new gene of the f1 operon of Y. pestis involved in the capsule biogenesis. FEBS Lett. 29777-80. [DOI] [PubMed] [Google Scholar]
- 30.Kauffman, A. S., M. J. Paul, and I. Zucker. 2004. Increased heat loss affects hibernation in golden-mantled ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287R167-R173. [DOI] [PubMed] [Google Scholar]
- 31.Klena, J., R. S. Ashford II, and C. A. Schnaitman. 1992. Role of Escherichia coli K-12 rfa genes and the rfp gene of Shigella dysenteriae 1 in generation of lipopolysaccharide core heterogeneity and attachment of O antigen. J. Bacteriol. 1747297-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klena, J., P. Zhang, O. Schwartz, S. Hull, and T. Chen. 2005. The core lipopolysaccharide of Escherichia coli is a ligand for DC-SIGN (CD209) receptor. J. Bacteriol. 1871710-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klena, J. D., and C. A. Schnaitman. 1993. Function of the rfb gene cluster and the rfe gene in the synthesis of O antigen by Shigella dysenteriae 1. Mol. Microbiol. 9393-402. [DOI] [PubMed] [Google Scholar]
- 34.Kolodziejek, A. M., D. J. Sinclair, K. S. Seo, D. R. Schnider, C. F. Deobald, H. N. Rohde, A. K. Viall, S. S. Minnich, C. J. Hovde, S. A. Minnich, and G. A. Bohach. 2007. Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis KIM. Microbiology 1532941-2951. [DOI] [PubMed] [Google Scholar]
- 35.Kutyrev, V., R. J. Mehigh, V. L. Motin, M. S. Pokrovskaya, G. B. Smirnov, and R. R. Brubaker. 1999. Expression of the plague plasminogen activator in Yersinia pseudotuberculosis and Escherichia coli. Infect. Immun. 671359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lathem, W. W., P. A. Price, V. L. Miller, and W. E. Goldman. 2007. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science 315509-513. [DOI] [PubMed] [Google Scholar]
- 37.Li, G., Y. J. Kim, C. Mantel, and H. E. Broxmeyer. 2003. P-selectin enhances generation of CD14+CD16+ dendritic-like cells and inhibits macrophage maturation from human peripheral blood monocytes. J. Immunol. 171669-677. [DOI] [PubMed] [Google Scholar]
- 38.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 3091739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 3001295-1297. [DOI] [PubMed] [Google Scholar]
- 40.Miller, V. L., and S. Falkow. 1988. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect. Immun. 561242-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morona, R., C. Daniels, and L. Van Den Bosch. 2003. Genetic modulation of Shigella flexneri 2a lipopolysaccharide O antigen modal chain length reveals that it has been optimized for virulence. Microbiology 149925-939. [DOI] [PubMed] [Google Scholar]
- 42.Murray, G. L., S. R. Attridge, and R. Morona. 2003. Regulation of Salmonella typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz. Mol. Microbiol. 471395-1406. [DOI] [PubMed] [Google Scholar]
- 43.Oyston, P. C., J. L. Prior, S. Kiljunen, M. Skurnik, J. Hill, and R. W. Titball. 2003. Expression of heterologous O-antigen in Yersinia pestis KIM does not affect virulence by the intravenous route. J. Med. Microbiol. 52289-294. [DOI] [PubMed] [Google Scholar]
- 44.Portnoy, D. A., and R. J. Martinez. 1985. Role of a plasmid in the pathogenicity of Yersinia species. Curr. Top. Microbiol. Immunol. 11829-51. [DOI] [PubMed] [Google Scholar]
- 45.Prior, J. L., J. Parkhill, P. G. Hitchen, K. L. Mungall, K. Stevens, H. R. Morris, A. J. Reason, P. C. Oyston, A. Dell, B. W. Wren, and R. W. Titball. 2001. The failure of different strains of Yersinia pestis to produce lipopolysaccharide O-antigen under different growth conditions is due to mutations in the O-antigen gene cluster. FEMS Microbiol. Lett. 197229-233. [DOI] [PubMed] [Google Scholar]
- 46.Riley, G., and S. Toma. 1989. Detection of pathogenic Yersinia enterocolitica by using Congo red-magnesium oxalate agar medium. J. Clin. Microbiol. 27213-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosqvist, R., M. Skurnik, and H. Wolf-Watz. 1988. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature 334522-524. [DOI] [PubMed] [Google Scholar]
- 48.Russo, T. A., B. A. Davidson, U. B. Carlino-MacDonald, J. D. Helinski, R. L. Priore, and P. R. Knight III. 2003. The effects of Escherichia coli capsule, O-antigen, host neutrophils, and complement in a rat model of Gram-negative pneumonia. FEMS Microbiol. Lett. 226355-361. [DOI] [PubMed] [Google Scholar]
- 49.Schnaitman, C. A., and J. D. Klena. 1993. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol. Rev. 57655-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sebbane, F., C. O. Jarrett, D. Gardner, D. Long, and B. J. Hinnebusch. 2006. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc. Natl. Acad. Sci. USA 1035526-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skurnik, M., and H. Wolf-Watz. 1989. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol. Microbiol. 3517-529. [DOI] [PubMed] [Google Scholar]
- 52.SolFoulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado, J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16145-155. [DOI] [PubMed] [Google Scholar]
- 53.Steeghs, L., U. Uronen-Hansson, S. Van Vliet, A. Van Mourik, N. Klein, Y. Van Kooyk, R. Callard, J. Van de Winkel, and P. Van der Ley. 2006. Lipopolysaccharide-mediated targeting of Neisseria meningitidis to dendritic cells: binding of lgtB LPS to DC-SIGN. Cell. Microbiol. 8316-325. [DOI] [PubMed] [Google Scholar]
- 54.Tailleux, L., N. Pham-Thi, A. Bergeron-Lafaurie, J. L. Herrmann, P. Charles, O. Schwartz, P. Scheinmann, P. H. Lagrange, J. de Blic, A. Tazi, B. Gicquel, and O. Neyrolles. 2005. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tailleux, L., O. Schwartz, J. L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3697-709. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez-Torres, A., and F. C. Fang. 2000. Cellular routes of invasion by enteropathogens. Curr. Opin. Microbiol. 354-59. [DOI] [PubMed] [Google Scholar]
- 58.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 5969-89. [DOI] [PubMed] [Google Scholar]
- 59.Weeks, S., J. Hill, A. Friedlander, and S. Welkos. 2002. Anti-V antigen antibody protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb. Pathog. 32227-237. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, J., G. Li, A. Bafica, M. Pantelic, P. Zhang, H. Broxmeyer, L. M. Wetzler, J. He, and T. Chen. 2005. Neisseria gonorrhoeae enhances the infection of dendritic cells by human immunodeficiency virus type-1 (HIV-1). J. Immunol. 1747995-8002. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, P., O. Schwartz, M. Pantelic, G. Li, Q. Knazze, C. Nobile, M. Radovich, J. He, S. C. Hong, J. Klena, and T. Chen. 2006. DC-SIGN (CD209) recognition of Neisseria gonorrhoeae is circumvented by lipooligosaccharide variation. J. Leukoc. Biol. 79731-738. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, P., S. Snyder, P. Feng, P. Azadi, S. Zhang, S. Bulgheresi, K. E. Sanderson, J. He, J. Klena, and T. Chen. 2006. Role of N-acetylglucosamine within core lipopolysaccharide of several species of Gram-negative bacteria in targeting the DC-SIGN (CD209). J. Immunol. 1774002-4011. [DOI] [PubMed] [Google Scholar]
- 63.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 932588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou, L. J., and T. F. Tedder. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 1543821-3835. [PubMed] [Google Scholar]