Abstract
Heterotrimeric G proteins (peripheral proteins) conduct signals from membrane receptors (integral proteins) to regulatory proteins localized to various cellular compartments. They are in excess over any G protein-coupled receptor type on the cell membrane, which is necessary for signal amplification. These facts account for the large number of G protein molecules bound to membrane lipids. Thus, the protein-lipid interactions are crucial for their cellular localization, and consequently for signal transduction. In this work, the binding of G protein subunits to model membranes (liposomes), formed with defined membrane lipids, has been studied. It is shown that although G protein α-subunits were able to bind to lipid bilayers, the presence of nonlamellar-prone phospholipids (phosphatidylethanolamines) enhanced their binding to model membranes. This mechanism also appears to be used by other (structurally and functionally unrelated) peripheral proteins, such as protein kinase C and the insect protein apolipophorin III, indicating that it could constitute a general mode of protein-lipid interactions, relevant in the activity and translocation of some peripheral (amphitropic) proteins from soluble to particulate compartments. Other factors, such as the presence of cholesterol or the vesicle surface charge, also modulated the binding of the G protein subunits to lipid bilayers. Conversely, the binding of G protein-coupled receptor kinase 2 and the G protein β-subunit to liposomes was not increased by hexagonally prone lipids. Their distinct interactions with membrane lipids may, in part, explain the different cellular localizations of all of these proteins during the signaling process.
Keywords: hexagonal phase, phosphatidylethanolamine, signal transduction, protein kinase C
G protein-coupled receptors form the largest and most versatile family of signal-transducing membrane proteins known (1). Signals transduced by heterotrimeric G proteins, from an agonist-activated receptor, modulate an effector system that controls the cellular concentrations of second messengers (cAMP, cGMP, diacylglycerol, or certain ions), which, in turn, regulate the activity of other enzymes or signaling proteins. The overall process results in an amplification cascade, whereby a single agonist-activated receptor activates several G protein molecules, and so on. Evidence suggests the importance of lipids in the interaction of G proteins (and possibly other amphitropic proteins) with membranes. First, the molar excess of G proteins on plasma membranes (most of them bound to membrane lipids) over any receptor type is necessary for the first amplification step in the transduction pathway (2). Second, these peripheral proteins can translocate from the plasma membrane to the cytosol, so that their interactions with lipids are of relevance to understand signal transduction mechanisms (3, 4). Finally, G proteins have been found to be associated with several soluble and particulate structures (5), and their localization and activity must, in part, be controlled by protein-lipid interactions. Here we have studied the interaction of the G protein α-subunits (Gαi1/2) (and other amphitropic proteins) with the major membrane lipids: phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and cholesterol (CHO), as well as the membrane surface charge. For this purpose model synthetic membranes (i.e., liposomes) rather than natural membranes were used, because synthetic membranes constitute a simple system where the lipid composition can be precisely controlled. Although Gα proteins (Gαi and Gαs) bind to lipid bilayers, the presence of nonbilayer-prone lipids, such as PEs, induced a significant and concentration-dependent increase in the binding of this protein to liposomes. Moreover, PEs with lower lamellar to hexagonal (HII) phase transition temperature were more effective in increasing this binding, indicating that the presence or propensity to form such nonlamellar structures may play an important role in the G protein association with membranes. Nonlamellar (HII) lipid structures are required for fusion and fission of lipid vesicles (6, 7) and for the activity of certain enzymes (8), but, in general terms, the reason these lipid species are so abundant in natural membranes remains unclear. Interestingly, this mechanism affects not only G proteins, but also other unrelated proteins [protein kinase C (PKC) and the insect peripheral protein apolipophorin III] (9). Apolipophorin III can be translocated from hemolymph to the cell membrane and exhibits two modes of interaction with membranes: a low-affinity binding to lamellar structures and a high-affinity binding to nonlamellar phases. Conversely, G protein-coupled receptor kinase 2 (GRK2) and G protein β-subunit (Gβ) binding to liposomes was not favored by PE. The presence of other lipids (PS and CHO) or the surface charge on PC bilayers also appeared to play an important and differential role in protein-lipid interactions. The present data, along with other several works, add evidence to extend the fluid mosaic model of the structure of cell membranes (10). This extended fluid mosaic model contemplates additional structural and functional aspects of membrane organization: the mosaic is configured, not only by different proteins on membranes, but also by (i) regions enriched in certain protein and lipid species, (ii) the different protein and lipid composition of outer and inner leaflets of the plasma membrane, and (iii) local (domain-restricted) membrane functions provided by the discrete localization of membrane proteins and lipids and by the lipid polymorphism.
MATERIALS AND METHODS
Peripheral Protein Extraction.
Rat brain cortex was used, because of the abundance of peripheral proteins involved in signal transduction (11, 12). For the extraction of these amphitropic proteins, four rat brain cortices (approximately 2.5 g of frontal cortex) were homogenized in 40 ml of 10 mM Hepes/250 mM sucrose/5 mM EDTA/5 mM EGTA/1 mM phenylmethylsulfonyl fluoride/5 mM iodoacetamide at pH 7.4. The membrane suspension was centrifuged at 800 × g for 10 min at 4°C. The supernatant was centrifuged at 3,000 × g for 10 min at 4°C. The resulting supernatant was incubated in the presence of 1 M NaCl for 30 min, at room temperature, then was centrifuged twice at 250,000 × g for 1 hr at 4°C, with the pellet discarded after each centrifugation. The final supernatant was extensively dialyzed against 10 mM Hepes buffer at pH 7.4 and stored at −80°C until use. Protein content was determined as described (13).
Formation of Liposomes.
Liposomes were formed similarly as described (14). Briefly, vesicles consisting of one or more lipid species were prepared by dissolving the lipids in chloroform and mixing appropriate volumes in glass vials. The solvent was removed under argon flux, and the residue was submitted to vacuum for at least 3 hr. The lipid film was resuspended in 2 ml of 10 mM Hepes/100 mM NaCl at pH 7.4 by vortex shaking and incubated 20 min at room temperature. Then, 3.3 ml of 10 mM Hepes buffer at pH 7.4 was added, and the mixture was incubated for 20 min at room temperature and used immediately for binding assays.
Binding Assays.
The liposome suspension, prepared as described above (5.3 ml), was combined with 0.7 ml of peripheral membrane protein suspension and incubated for 30 min at room temperature. The final conditions of the assay were: 500 μM of lipid phosphorus, 500 μg of proteins, 10 mM Hepes, and 33 mM NaCl at pH 7.4, in a total volume of 6 ml. This mixture was centrifuged at 100,000 × g for 1 hr at 25°C. Pellets were resuspended in 180 μl of 80 mM Tris⋅HCl/4% SDS at pH 6.8, and incubated for 10 min at room temperature. The lipid phosphorus concentration was determined as described (15). Then, 20 μl of 120 mM Tris⋅HCl/50% glycerol/4% SDS/58 mM β-mercaptoethanol/10 mM N-ethylmaleimide/0.2% bromophenol blue at pH 6.8 was added, and the mixture was boiled for 3 min.
SDS/PAGE and Immunoblotting.
Peripheral proteins bound to the liposome surface were detected and quantitated by means of specific antibodies, similarly as described (11, 16). Briefly, 5–40 μl of protein-lipid suspensions of natural or synthetic PC species (about 75–600 nmol of lipid phosphorus) were loaded on 10% polyacrylamide gels (SDS/PAGE). Duplicate aliquots of 15 and 25 μl of lipid-protein suspensions (225 and 375 nmol of lipid phosphorus, respectively) from test samples (other than PC liposomes) were loaded on the same gel. After electrophoresis, proteins were transferred onto nitrocellulose membranes (immunoblotting), which were incubated for 1 hr at room temperature in PBS containing 5% nonfat dry milk, 0.5% BSA, and 0.1% Tween 20 (blocking solution) (17). The membranes subsequently were incubated overnight at 4°C in fresh blocking solution containing the primary antibody (anti-Gαi1/2 antiserum, 1:5,000 dilution; anti-Gαs antiserum, 1:3,000 dilution; anti-Gβ antiserum, 1:5,000 dilution; anti-PKC-α/β antibody, 1:2,000 dilution; and anti-GRK2 antiserum, 1:3,000 dilution). The secondary antibody, horseradish peroxidase-linked donkey anti-rabbit (G proteins, GRK2, 1:5,000 dilution) or sheep anti-mouse (PKC, 1:5,000 dilution) IgG was incubated for 2 hr at room temperature in fresh blocking solution. Immunoreactivity was detected by a chemiluminescence procedure (ECL, Amersham). After autoradiography, the films were scanned, and the integrated optical density values were obtained by image analysis (Bio Image, Millipore). For the quantitation of the bound protein, four test samples were evaluated using standard PC curves, consisting of five points of different lipid phosphorus content. This procedure was performed twice in the same experiment for each test sample. Each experiment was repeated at least three times. Further details about these procedures have been described elsewhere (11).
Materials.
Anti-G protein antibodies (anti-Gαi1/2, anti-Gαs, and anti-Gβ) were purchased from DuPont/NEN. Anti-PKCα/β antibody, horseradish peroxidase-linked IgGs, enhanced chemiluminescence reagents (ECL Western Blot Detection System), and ECL hyperfilm (autoradiography film) were purchased from Amersham. Anti-GRK2 was a kind gift from Federico Mayor, Jr. (Universidad Autónoma, Madrid, Spain). Lipids CHO, dioleoyl phosphatidylethanolamine, dipalmitoyl phosphatidylcholine (DPPC), 1-palmitoyl-2-linoleoyl phosphatidylethanolamine, and 1-palmitoyl-2-oleoyl phosphatidylethanolamine (POPE) were purchased from Sigma. Egg PC, egg PE, bovine brain PS, dielaidoyl phosphatidylethanolamine, and positive and negative charge liposome kits were purchased from Avanti Polar Lipids.
Statistics.
Results are expressed as mean ± SEM. One-way ANOVA followed by Scheffé’s test was used for statistical evaluations. The level of significance was chosen as P = 0.05.
RESULTS
Effect of PE on the Binding of Gαi to Model Membranes.
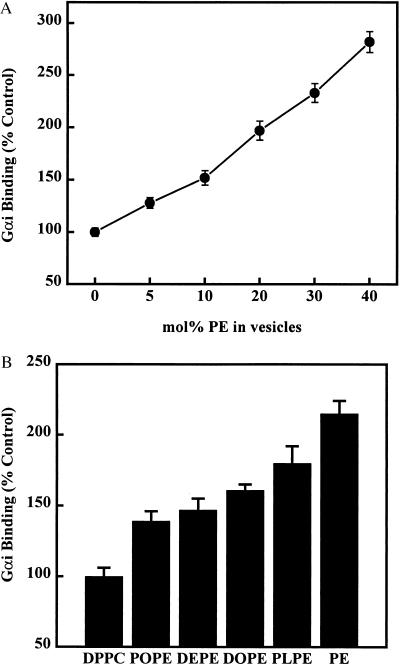
Some lipids, such as PEs, favor the formation of nonlamellar (HII) membrane structures (Fig. 1). The presence of egg PE in egg PC liposomes induced, at all concentrations used, significant and concentration-dependent increases in the binding of Gαi to lipid vesicles at 25°C (about a 3-fold increase for PC/PE 6:4, mol/mol, respect to PC liposomes) (Figs. 2A and 3). In DPPC vesicles, the presence of natural and synthetic PE species (DPPC/PE 6:4, mol/mol) also induced increases in Gαi binding (Figs. 2B and 3). Moreover, PE species with a higher propensity to form HII phases at 25°C (e.g., egg PE and 1-palmitoyl-2-linoleoyl phosphatidylethanolamine) produced greater increases in Gαi binding than those of PE species with lesser propensity to induce HII structures [e.g., POPE and dielaidoyl phosphatidylethanolamine, which exhibit higher lamellar to hexagonal phase transition temperatures (TH)]. This effect is due either to the coexistence of lamellar and nonlamellar structures (18) or to the increased HII propensity. When the same PE species were mixed with egg PC, instead of DPPC, a similar enhancement of Gαi binding was observed (data not shown). Some PE species used in these series of experiments were assayed at various PC/PE molar ratios, and there were also concentration-dependent increases in Gαi binding to liposomes (e.g., 15 mol% of POPE or dielaidoyl phosphatidylethanolamine in PC vesicles induced increases of 21% and 23%, respectively, and 40 mol% of these PEs induced increases of 39% and 47%, respectively). These data clearly indicate that the presence of nonlamellar-prone lipids favors the binding of Gαi to membranes.
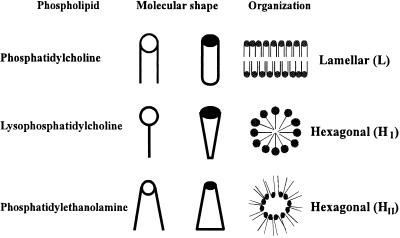
Figure 1.
Lipid shape and supramolecular organization (polymorphism). The lipid shape is a very convenient concept, frequently used to describe the volume occupied by phospholipids. Using this approach, phospholipids can be classed as cylinders (e.g., PC), cones (e.g., PE) and inverted cones (e.g., lysophosphatiodylcholine), depending on the relative volumes of their polar headgroups and fatty acyl chains. The supramolecular organization, or packing, of such molecules originates the widespread bilayer (or lamellar, L) structure, and the nonlamellar (tubular micelles) HI and HII phases.
Figure 2.
Effect of PE on the binding of Gαi to liposomes. (A) Liposomes were formed with egg PC without (0 mol% PE) or with increasing amounts of egg PE (5–40 mol% PE). (B) Liposomes were formed with DPPC, and egg or synthetic PE derivatives. The PC/PE molar ratio was 6:4 in all cases, although for each PE species, various concentrations were tested, similarly as shown in A (see text). The phospholipid concentration was 500 μM in all cases (A and B), and the total protein content was 500 μg. The binding of Gαi to all PE-containing liposomes (A and B) appeared to be significantly different from that to PC liposomes (P < 0.01). DEPE, dielaidoyl phosphatidylethanolamine; DOPE, dioleoyl phosphatidylethanolamine; PLPE, 1-palmitoyl-2-linoleoyl phosphatidylethanolamine.
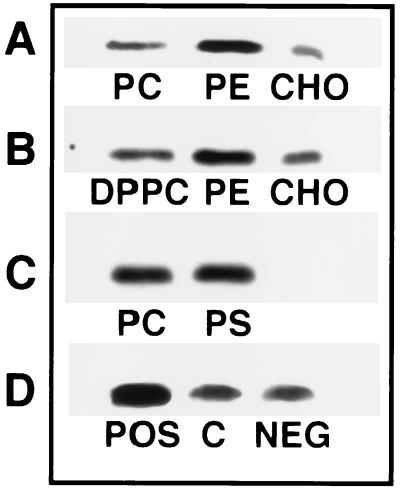
Figure 3.
Representative immunoblots of the binding of Gαi protein to membrane lipids. (A) Binding to egg PC vesicles in the absence (PC) or presence of egg PE (PC/PE 6:4, mol/mol) or CHO (PC/CHO 1:1, mol/mol). (B) Same as A, but with DPPC instead of egg PC. (C) Immunoreactive Gαi protein bound to egg PC vesicles in the absence (PC) or presence of bovine brain PS (PS, PC/PS 4:1, mol/mol). (D) Binding of Gαi to egg PC/CHO liposomes (PC/CHO 7:1, mol/mol) in the absence (C, control) or presence of 143 μM of stearylamine (POS, positively charged vesicles) or dicetyl phosphate (NEG, negatively charged vesicles).
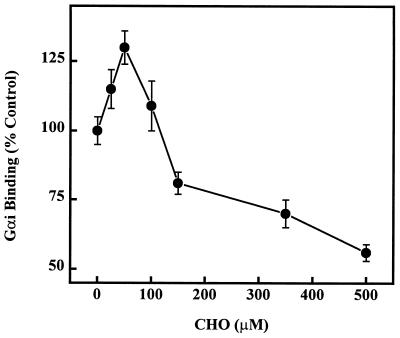
Effect of CHO on the Binding of Gαi to Model Membranes.
The binding of Gαi to PC liposomes (500 μM) with increasing amounts of CHO exhibited a biphasic behavior (Figs. 3 and 4). For low CHO concentrations (25–50 μM, 5–10 mol%) a moderate, but significant, increase (15–30%, P < 0.05) in the binding of Gαi to lipid vesicles was observed (Fig. 4). Higher CHO concentrations (30–50 mol%), induced significant decreases (19–44%, P < 0.01) in the binding of this protein to PC vesicles (Fig. 4). In another series of experiments, mixtures of either egg PC or DPPC and various PE species, in the presence or absence of CHO, were used. In these cases, the presence of 500 μM CHO also induced significant decreases in Gαi binding to lipid vesicles: 44%, 41%, 38%, and 25% decreases for egg PC, egg PC/PE, egg PC/POPE, and egg PC/dioleoyl phosphatidylethanolamine (PC/PE species 3:7, mol/mol in all cases), respectively; 49%, 53%, and 64% decreases for DPPC, DPPC/PE, and DPPC/dioleoyl phosphatidylethanolamine (PC/PE species 3:7, mol/mol in all cases), respectively.
Figure 4.
Effect of CHO on the binding of Gαi to liposomes. Liposomes contained 500 μM of egg PC in the absence (0 μM CHO) or presence of various CHO concentrations (25–500 μM). The binding of Gαi to liposomes containing 25–50 μM CHO was significantly higher than that to PC vesicles (P < 0.05), whereas the binding to vesicles containing 100–500 μM CHO was significantly lower (P < 0.01).
Effects of PS and the Vesicle Surface Charge on the Binding of Gαi to Model Membranes.
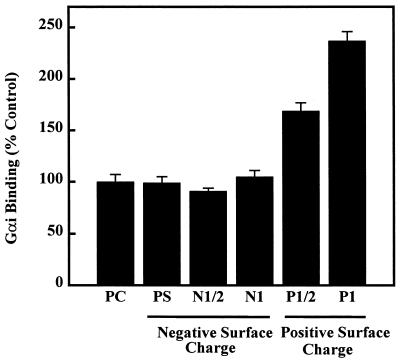
The presence of PS (negative surface charge) in egg PC liposomes (PC/PS 19:1 to 6:4, mol/mol) did not induce significant changes in Gαi binding to lipid vesicles (100%, 94%, 102%, 107%, and 99% for 0, 5, 10, 20, and 40 mol% PS, respectively; Figs. 3 and 5). In a similar fashion, the presence of a net negative surface charge, provided by dicetyl phosphate, on PC liposomes, did not alter the Gαi binding to lipid vesicles (Figs. 3 and 5). Conversely, the binding of PKC to PS-containing and to negatively charged liposomes was significantly higher than that to PC liposomes (408% and 282%, respectively). The interaction of Gαi and PKC with negatively charged liposomes revealed a significant difference between these two peripheral proteins, which may, in part, explain their differential localization during the signaling cycle. On the other hand, a positive surface charge induced a marked increase in Gαi binding to PC bilayers (Figs. 3 and 5).
Figure 5.
Effect of PS and the membrane surface charge on the Gαi binding to liposomes. Egg PC liposomes in the absence (PC) or presence of PS (PS, PC/PS 6:4, mol/mol) (negative surface charge), dicetyl phosphate (negative surface charge, N1/2, 71 μM; N1, 143 μM), or stearylamine (positive surface charge, P1/2, 71 μM; P1, 143 μM). The binding of Gαi to negatively charged liposomes was not significantly different from that to PC liposomes (P > 0.05), but the binding to positively charged vesicles was significantly increased (P < 0.01).
Effect of Lipid Polymorphism on the Binding of Gβ to Model Membranes.
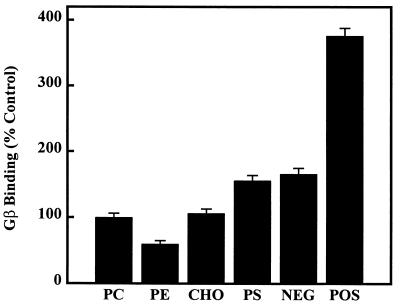
The G protein β-subunit (or the Gβ/γ complex) showed a pattern of interaction with lipid vesicles different from that of Gαi-subunits. Thus, the binding of Gβ to liposomes (500 μM phosphorus lipid) was decreased by PE (40% decrease, egg PC/egg PE 6:4, mol/mol, P < 0.01) (Fig. 6). On the other hand, CHO did not alter its interaction with PC bilayers, and a negative surface charge (40 mol% of PS or 25 mol% of dicetyl phosphate) induced increases of Gβ binding (56% and 66%, respectively, P < 0.01) (Fig. 6). Finally, the presence of a positive surface charge (25 mol% of stearylamine) on membranes markedly increased the binding of Gβ-subunits (about 4-fold increase, P < 0.01) (Fig. 6).
Figure 6.
Gβ binding to liposomes. Vesicles were formed with egg PC in the absence (PC) or presence of egg PE (PE, PC/PE 6:4, mol/mol), 200 μM CHO, bovine brain PS (PS, PC/PS 6:4, mol/mol), a negative (NEG, 143 μM dicetyl phosphate), or a positive (POS, 143 μM stearylamine) surface charge. In all cases the phospholipid concentration was 500 μM.
Effect of PE on the Binding of Various Peripheral Membrane Proteins.
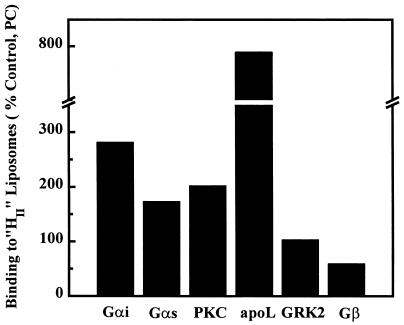
As shown above, the presence of nonlamellar-prone lipids induced an increase in the binding of Gαi proteins to PC vesicles. Gαs and PKC also displayed an enhanced binding to PC/PE (6:4, mol/mol) liposomes compared with that to PC vesicles (174% and 282%, respectively) (Fig. 7). In addition to these signaling proteins, the insect peripheral protein, apolipophorin III, has been shown to display an enhanced binding to liposomes containing nonlamellar-prone lipids (9). Other peripheral proteins, however, did not exhibit this behavior: the binding of GRK2 to liposomes was not altered by the presence of PE, and that of Gβ was decreased (40%, Fig. 7). These data suggest that the augmentation of the HII phase propensity could be a general mechanism for the interaction of various peripheral proteins with the membrane lipids, which might be relevant for the localization, trafficking, and activity of such proteins.
Figure 7.
Effect of nonlamellar-prone lipids on the binding of various peripheral proteins to model membranes. The results are expressed as percent change respect to the binding to the bilayer-prone lipid, PC (100%). Gαi protein (Gαi), Gαs protein (Gαs), PKC, and apolipophorin III (apoL) were found to have an enhanced binding to liposomes containing HII-prone lipids. GRK2 did not show any difference for the binding to PC in the absence or presence of PE (104% for PC/PE 6:4, mol/mol, respect to PC liposomes). Gβ-subunits showed a reduced binding to PE-containing liposomes. The value for apoL was taken from Soulages et al. (9).
DISCUSSION
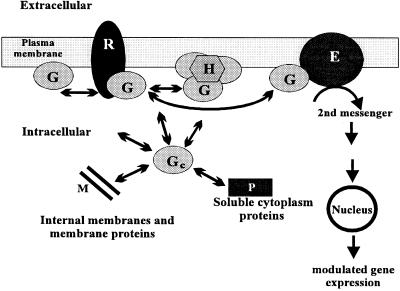
Receptor-mediated transduction pathways operate as signal amplification cascades. The first amplification step happens at the receptor-G protein level, whereby a molecule of the former activates several of the latter upon agonist binding (2). Because many receptors do not appear to be homogeneously distributed on the cell surface, but clustered in defined membrane regions (e.g., the synaptic cleft), a large number of inactive (i.e., GDP-bound) G protein molecules must be available in the receptor vicinity, most of them probably associated with membrane lipids (Fig. 8). Some lipids, such as PEs, are able to array in vitro into structures different from the bilayer organization that characterizes biological membranes (Fig. 1) (10). In the present study it has been shown that the presence of the nonlamellar-prone lipid PE favors the binding of G proteins to model membranes (Figs. 2, 3, and 8). Cell membranes are usually rich in PE, which primarily is distributed on the protoplasmic face of the plasma membrane (19). The presence of PE-rich domains or nonlamellar regions, either in a stable or transient manner, could act as membrane reservoirs for some peripheral proteins (Fig. 8). Because those PE species with higher propensity to induce HII phases (18) also induced higher increases in the binding of G proteins to liposomes (Fig. 2), this interaction could be related to the HII phase propensity or to the presence of nonlamellar structures (possibly stabilized by the presence of peripheral/amphitropic proteins). This hypothesis is in agreement with the observed loss of G proteins and PKC from brain cortex plasma membranes after disruption of nonlamellar HII structures by the anthracycline daunomycin (16). Nonlamellar-prone lipids alter some physicochemical properties of lipid bilayers, inducing membrane “defects” (9), such as changes in the intrinsic monolayer curvature, the surface pressure, and the hydration of membranes. The relative amounts of PEs on membranes, under physiological conditions, could justify the possible in vivo presence of HII structures (18) that might be stabilized by peripheral/amphitropic proteins. Moreover, the transient presence of such HII phases or other membrane defects may contribute to regulate certain cellular events, such as peripheral protein trafficking, exo-endocytic processes, and vesicle fusion/fission phenomena. Some recent studies support the involvement of nonlamellar structures, rather than the alteration of other physicochemical properties of membranes, in the control of a number of functions: (i) when PEs are induced to adopt the lamellar phase, G proteins and PKC dissociate from brain cortex plasma membranes (16); (ii) the binding of the insect peripheral protein apolipophorin III to model membranes also appears to be favored by the presence of HII structures (9); (iii) PE species with lower hexagonal phase transition temperature (TH) values also induce greater increases in G protein binding (this work) and in the PKC activity and binding to membranes (20); and (iv) PEs in the hexagonal phase, but not in the lamellar phase, are able to modulate the Ca2+-ATPase activity (8). The present study documents an important role of hexagonal-prone lipids (and possibly of HII propensity or HII structures) for signal transduction and other cellular events involving peripheral proteins whose interaction with membranes is regulated by nonlamellar-prone lipids. These results, along with other studies, extend the knowledge about the cell membrane structure and function, showing aspects of membrane organization, relevant in the context of the membrane fluid mosaic model (10). In this line, neural membranes of cold-adapted fishes show increased levels of PE species that lower the TH value (21), suggesting that the hexagonal phase propensity plays a pivotal role in membrane phenomena. Furthermore, important cellular events in which PEs (or nonlamellar membrane structures) are involved, such as membrane fusion (7), PEs redistribution at the cleavage furrow of dividing cells during cytokinesis (22), and peripheral protein binding facilitation (this work), may explain the high proportion of this phospholipid species (PE) in most plasma membranes.
Figure 8.
Interaction of G proteins (G) with different cellular structures. The cytoplasmic face of the lipid bilayer contains high amounts of G proteins and of the HII phase-prone lipid PE, which may induce the presence of nonlamellar structures or other membrane “defects” (H). G proteins also can interact with other membrane components, such as bilayer-preferring lipids, G protein-coupled receptors (R), and signaling effectors (E). They also can translocate to the cytoplasm, where they are found in the soluble fraction and associated with internal membranes, interacting with their lipids or proteins. Extracellular or intracellular signals acting on G protein-coupled receptors can change the proportions of these populations, which coexist in a dynamic equilibrium, also regulated, as shown here, by the membrane lipid structure.
On the other hand, CHO, another major lipid component of biological membranes has been shown to modulate the physicochemical properties of lipid bilayers: it alters the membrane fluidity, facilitates the formation of nonlamellar structures, and induces the lipid segregation (23, 24). Because CHO can move rapidly from one membrane to another, it also can act as a short-term regulator of the membrane structure and function. Interestingly, the effect of this lipid on the interaction of Gαi with PC bilayers was not homogeneous over the range of CHO concentrations used (Fig. 4). At low concentrations (up to around 15–20% of the total membrane lipids), CHO favored the binding of Gα proteins to lipid bilayers, but at higher concentrations (30–50 mol% CHO) it impaired this binding. The ability of this lipid to induce nonlamellar structures at low mol fractions (less than 0.2) and inhibit them at higher mol fractions would explain the biphasic behavior of the Gαi binding to CHO-containing membranes (25). On the other hand, CHO intercalates in the surface of the lipid leaflet, a potential target for the binding of the G protein fatty acid moiety, which favors the interaction between G proteins and membranes (26, 27). High CHO concentrations may compete with G proteins for these sites on the membrane. Because CHO can induce the formation of CHO-rich and CHO-poor membrane domains (24), it cannot be discarded that both effects (i.e., concentration-dependent increases or decreases in Gαi binding) may operate simultaneously on biological membranes.
The presence of PS (negative surface charge) on liposomes induced a marked increase in the binding of PKC (about 4-fold increase for PC/PS 6:4, mol/mol), but Gαi binding to lipid bilayers was not significantly altered. Similarly, dicetyl phosphate, which also provides a negative surface charge, did not modify the binding of Gαi to lipid vesicles. In contrast, a positive surface charge increased Gαi binding (Fig. 5). The inner leaflet of the plasma membrane contains high levels of PS and has a negative surface charge. For this reason, it is unlikely that electrostatic interactions involving positive charges were relevant in the interaction of Gαi with the plasma membrane phospholipids, although the electrostatic interactions may play an important role in Gαi’s interaction with membrane proteins or with other cell membranes. The differential interactions of G proteins and PKC with membrane lipids may, in part, explain why the inactive form of PKC is mainly found in the cytosol, whereas the inactive form of Gαi prefers the plasma membrane (3, 28, 29).
The pattern of interaction of Gβ subunits (Gβ/γ complex under the reported experimental conditions) with model membranes was different to that of Gαi subunits. CHO did not induce significant alterations, and PE induced a decrease in Gβ binding to lipid bilayers. Interestingly, both negative and positive surface charges (greater effect) facilitated the interaction of this protein with lipid vesicles, indicating that electrostatic forces may drive the association of Gβ with membranes. The differences in protein-lipid interactions between G protein α- and β-subunits might be involved in their divergent cellular localizations after the G protein complex dissociation, induced by the binding of GTP (30).
In summary, protein-lipid interactions play a pivotal role in the association of peripheral (amphitropic) proteins with membranes. Because G proteins, PKC (this work), and apolipophorin III (9) are structurally and functionally unrelated peptides, the amphitropic protein-membrane binding facilitation induced by PEs could constitute a widespread protein-lipid interaction mechanism. However, the nature of the interactions of G protein subunits, PKC, and other peripheral proteins with phospholipids capable to form inverted micelles (i.e., negative intrinsic monolayer curvature strain) in vitro requires further investigations. Posttranslational modifications, or noncovalently bound lipids present on these proteins, could contribute to these interactions: Gα have a covalently bound palmitic and/or myristic acid molecule, γ-subunits have a farnesyl or geranylgeranyl moiety (29), and PKC has binding sites for diacylglycerol and PS (3). On the other hand, it cannot be discarded that the presence of these proteins on the plasma membrane may induce a stabilization of such nonlamellar structures. The proposed mechanism, however, does not account for the interaction of all peripheral proteins with membranes: GRK2 is able to translocate from microsomal membranes to other cellular compartments (31), but its binding to membranes was not modulated by PEs. It has been proposed that this protein probably is anchored to a microsomal membrane protein (4, 32). In this context, the different cellular localization and interaction with membrane lipids of GRK2 (mainly on microsomal membranes), G proteins (mainly on plasma membranes), and PKC (mainly in the cytosol) further supports the involvement of PEs in the interaction of amphitropic proteins with membranes, although protein-protein interactions also play an important role in the cellular localization and activity of most of the known (amphitropic/peripheral) kinases (33).
Acknowledgments
This study was supported by Dirección General de Investigación Cientifica y Tecnica Grant PB94–0002-Mod C, Spain. J.A.G.-S. is a Member of the Institut d’Estudis Catalans.
ABBREVIATIONS
- CHO
cholesterol
- DPPC
dipalmitoyl phosphatidylcholine
- Gα
G protein α-subunit
- Gβ
G protein β-subunit
- GRK2
G protein-coupled receptor kinase 2
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PKC
protein kinase C
- PS
phosphatidylserine
- POPE
1-palmitoyl-2-oleoyl phosphatidylethanolamine
- HI and HII
hexagonal I and II
References
- 1.Dohlman H G, Thorner J, Caron M G, Lefkowitz R J. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen S E, Ross E M. Proc Natl Acad Sci USA. 1982;79:7228–7232. doi: 10.1073/pnas.79.23.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishizuka Y. Nature (London) 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 4.García-Higuera I, Penela P, Murga C, Egea G, Bonay P, Benovic J L, Mayor F., Jr J Biol Chem. 1994;269:1348–1355. [PubMed] [Google Scholar]
- 5.Lewis J M, Woolkalis M J, Gerton G L, Smith R M, Jarret L, Manning D R. Cell Regul. 1991;2:1097–1113. doi: 10.1091/mbc.2.12.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seddon J M. Biochim Biophys Acta. 1990;1031:1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 7.Wilschut J. In: Membrane Fusion. Wilschut J, Hoekstra D, editors; Wilschut J, Hoekstra D, editors. Groningen: Dekker; 1990. pp. 89–126. [Google Scholar]
- 8.Starling A P, Dalton K A, East J M, Oliver S, Lee A G. Biochem J. 1996;320:309–314. doi: 10.1042/bj3200309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soulages J L, Salamon Z, Wells M A, Tollin G. Proc Natl Acad Sci USA. 1995;92:5650–5654. doi: 10.1073/pnas.92.12.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer S J, Nicholson G L. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 11.Escribá P V, Sastre M, García-Sevilla J A. Arch Gen Psychiatry. 1994;51:494–501. doi: 10.1001/archpsyc.1994.03950060058006. [DOI] [PubMed] [Google Scholar]
- 12.Busquets X, Escribá P V, Sastre M, García-Sevilla J A. J Neurochem. 1995;64:247–252. doi: 10.1046/j.1471-4159.1995.64010247.x. [DOI] [PubMed] [Google Scholar]
- 13.Bradford M. Anal Biochem. 1976;72:248–252. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.Escribá P V, Ferrer-Montiel A V, Ferragut J A, González-Ros J M. Biochemistry. 1990;29:7275–7282. doi: 10.1021/bi00483a017. [DOI] [PubMed] [Google Scholar]
- 15.Kyaw A, Maung-U K, Toe T. Anal Biochem. 1985;145:230–234. doi: 10.1016/0003-2697(85)90354-9. [DOI] [PubMed] [Google Scholar]
- 16.Escribá P V, Sastre M, García-Sevilla J A. Proc Natl Acad Sci USA. 1995;92:7595–7599. doi: 10.1073/pnas.92.16.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 18.Perkins W R, Dause R B, Parente R A, Minchey S R, Neuman K C, Gruner S M, Taraschi T F, Janoff A S. Science. 1996;273:330–332. doi: 10.1126/science.273.5273.330. [DOI] [PubMed] [Google Scholar]
- 19.Cullis P R, Hope M J. In: Biochemistry of Lipids, Lipoproteins, and Membranes. Vance D E, Vance J, editors; Vance D E, Vance J, editors. Amsterdam: Elsevier; 1991. pp. 1–41. [Google Scholar]
- 20.Giorgione J, Epand R M, Buda C, Farkas T. Proc Natl Acad Sci USA. 1995;92:9767–9770. doi: 10.1073/pnas.92.21.9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buda C, Dey I, Balogh N, Norvath L I, Maderspach K, Juhasz M, Leo Y K, Farkas T. Proc Natl Acad Sci USA. 1994;91:8234–8238. doi: 10.1073/pnas.91.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, Umeda M. Proc Natl Acad Sci USA. 1996;93:12867–12872. doi: 10.1073/pnas.93.23.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloch K. In: Biochemistry of Lipids, Lipoproteins, and Membranes. Vance D E, Vance J, editors; Vance D E, Vance J, editors. Amsterdam: Elsevier; 1991. pp. 363–381. [Google Scholar]
- 24.Mabrey S, Mateo P L, Sturtevant J L. Biochemistry. 1978;17:2464–2470. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- 25.Cheetham J J, Wachtel E, Bach D, Epand R M. Biochemistry. 1989;28:8928–8934. doi: 10.1021/bi00448a036. [DOI] [PubMed] [Google Scholar]
- 26.Buss J E, Mumby S M, Casey P J, Gilman A G, Selfon B M. Proc Natl Acad Sci USA. 1987;84:7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones T L Z, Simonds W F, Merendino J J, Jr, Brann M R, Spiegel A M. Proc Natl Acad Sci USA. 1990;87:568–572. doi: 10.1073/pnas.87.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birnbaumer L. Annu Rev Pharmacol Toxicol. 1990;30:675–705. doi: 10.1146/annurev.pa.30.040190.003331. [DOI] [PubMed] [Google Scholar]
- 29.Wedegaertner P B, Wilson P T, Bourne H R. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- 30.Clapham D E, Neer E J. Nature (London) 1993;365:403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- 31.Sterne-Marr R, Benovic J L. Vitam Horm. 1995;51:193–234. doi: 10.1016/s0083-6729(08)61039-0. [DOI] [PubMed] [Google Scholar]
- 32.Murga C, Ruiz-Gómez A, García-Higuera I, Kim C M, Benovic J L, Mayor F., Jr J Biol Chem. 1996;271:985–994. doi: 10.1074/jbc.271.2.985. [DOI] [PubMed] [Google Scholar]
- 33.Mochly-Rosen D. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]