Abstract
Despite the fact that extensive evidence supports the view that phases of de novo protein synthesis are necessary for memory formation and maintenance, doubts are still raised. Skeptics generally argue that amnesia and the disruption of long-term synaptic plasticity are caused by “non-specific effects” of the reagents or approaches used to disrupt protein synthesis. This paper attempts to clarify some of these issues by reviewing, discussing and providing results addressing some of the major critiques that argue against the idea that de novo protein synthesis is necessary for the stabilization of long-term memory.
Introduction
During the last 40 years, numerous studies have provided evidence indicating that the formation of long-term memory and long-term synaptic plasticity requires protein synthesis. From the initial findings in the 1960s until now, hundreds of publications have reported that, in several species and a multitude of learning paradigms, a temporally limited treatment with protein synthesis inhibitors before or shortly after training produces amnesia (rev. in Davis & Squire 1984). Importantly, the same treatment at later times after training is ineffective, suggesting that memory formation depends upon an initial and temporally limited phase of protein synthesis. Moreover, even an established memory, which has become insensitive to the action of protein synthesis inhibitors can again return to a transient state of vulnerability if reactivated, for example by retrieval (rev in Lewis, 1979; Nader, Schafe, & LeDoux, 2000; Rudi, Biedenkapp, Moineau, & Bolding, 2006). The temporally limited requirement of protein synthesis after training seems to parallel the initial phase of memory consolidation, a process that indicates that memory is initially in a labile state, but over time becomes stable and resilient to disruptive interferences that include, in addition to protein synthesis inhibitors, trauma, seizure, brain cooling, RNA synthesis inhibition and additional learning (rev in: Alberini, 2005; Bailey, Bartsch, & Kandel, 1996; Dudai, 2004; Frankland & Bontempi, 2005; Gold, 2006; McGaugh, 2000; Squire & Alvarez, 1995). By analogy, because it is responsive to similar disruptive interferences, the process of re-stabilization of a memory that underwent reactivation is known as memory reconsolidation (Nader et al., 2000; Sara, 2000).
The finding that protein synthesis is necessary for memory consolidation and reconsolidation has fundamentally influenced and shaped the research aimed at understanding the molecular bases of learning and memory during the last 40–50 years. Many questions were asked following the initial discoveries: what are the proteins required for memory formation? In which brain regions are they necessary? For how long? In which subcellular compartment of the neuron is protein synthesis essential? What is the time course of protein synthesis requirements? Experiments that have and still are in the process of addressing these questions are leading to important new levels of understanding of how memory works. However, some issues concerning the validity of results and conclusions provided by these investigations, particularly those obtained with protein synthesis inhibitors, have been and continue to be the object of recurrent debates.
Some authors still question whether protein synthesis is truly essential during memory consolidation and reconsolidation, and generally put forward two main critiques. First, they propose that the amnesia produced by protein synthesis inhibitors is a result of “side effects” rather than inhibition of protein synthesis per se. Second, because in some cases the amnesia was found to be transient, and memory recovered after some time, some investigators hypothesize that the inhibitors’ effects target memory retrieval processes rather than memory consolidation or storage. Here, I will attempt to revisit, and hopefully help clarifying these two issues by discussing what it is known about the protein synthesis inhibitors “side effects“ and the effect of time on protein synthesis inhibition. I will also comment on additional evidence that, in my view, support the hypothesis that protein synthesis is indeed essential for memory consolidation and reconsolidation.
2. Protein synthesis inhibitors: mechanisms of action and side effects
Protein synthesis inhibitors widely used in long-term plasticity and memory investigations include anisomycin, cycloheximide, emetine and puromycin.
The mechanism of action of each of these inhibitors is distinct. Anisomycin {3,4- Pyrrolidinediol, 2-[(4-methoxyphenyl)methyl]-, 3-acetate, (2R,3S,4S)-)} is an antibiotic produced by Streptomyces griseolus that reversibly inhibits translation by binding to 60S ribosomal subunits and blocking peptide bond formation, thereby preventing elongation and causing polysome stabilization (Barbacid & Vasquez, 1975; Jimenez & Vasquez, 1979). Cycloheximide, 4-{(2R)-2-[(1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl]-2-hydroxyethyl} piperidine-2,6-dione, produced by the bacterium Streptomyces griseus, reversibly inhibits the translocation of aminoacyl-tRNA from the acceptor to the donor site in eukaryotic cells. The site is related to peptidyltransferase activity and appears to be a region on the larger ribosomal subunit (Obrig, Culp, McKeehan, & Hardesty, 1971; Rao & Grollman, 1967). Emetine is produced by Streptomyces alboniger and irreversibly blocks protein synthesis in both prokaryotes and eukaryotes by inhibiting the movement of ribosomes along the mRNA. Finally, puromycin (S)-3′;-[[2-amino-3-(4-methoxy-phenyl)-1-oxopropyl]amino]-3′-deoxy-N,N-dimethyl-adenosine, leads to the premature release of unfinished polypeptide chains as polypeptidyl-puromycin derivatives (Barbacid & Vasquez, 1975; Jimenez & Vasquez, 1979). This takes place because part of the puromycin molecule resembles the 3′ end of the aminoacylated tRNA and, as such, it enters the A site and transfers to the growing chain, causing premature chain release.
In addition to blocking protein synthesis, each of these inhibitors can also exert other actions, which have been suggested by some authors to potentially be the cause or largely contribute to the amnesia observed when they are administered following either initial learning or memory reactivation (Flexner & Goodman, 1975; Routtenberg & Rekart, 2005; Rudy et al., 2006). Indeed, as with any pharmacological compound, protein synthesis inhibitors do exhibit side effects, and different side effects manifest at different doses. Hence, it is important to keep in mind that, in order to determine whether or not the results obtained with protein synthesis inhibitors in learning and memory experiments are specific or due to side effects, dosage and relative side effects should be specified. Here I will review a number of side effects found at various doses, which include: partial inhibition of DNA synthesis, decrease in catecholamine biosynthesis, activation of kinases with superinduction of immediate early genes (IEGs) and apoptosis.
The partial inhibition of DNA synthesis has been found to be common to anisomycin, cycloheximide and emetine (Bennett, Smithers, & Ward, 1964; Grollman, 1967; Holloway & Ripley, 1952; Lieberman, Abrams, Hunt, & Ove, 1963; Mueller, Kajiwara, Stubblefield, & Rueckert, 1962). However, as pointed out in a number of reports, such inhibition of DNA synthesis probably results from a primary effect on protein synthesis, as the latter is required for the concurrent synthesis of DNA in animal cells (Grollman, 1967). In other words, this effect appears to be secondary to translation arrest.
A decrease in catecholamine accumulation has been viewed as a common effect of protein synthesis inhibitors as, in fact, it was reported with anisomycin, cycloheximide and puromicin (Flexner & Goodman, 1975). Hence this effect is often referred to as the major potential contributor to the non-translational side effects that may cause amnesia. The decrease in catecholamine was suggested to result from inhibition of tyrosine hydroxylase and acetyl-cholinesterase activities. Specifically, Flexner and Goodman (1975) reported that treatment with either cycloheximide, anisomycin or acetoxycycloeximide reduced the rate of accumulation of both norepinephrine and dopamine, whereas puromycin reduced only that of norepinephrine. Hence, they suggested that: these results weaken the assumption that the amnestic effects are due solely to inhibition of protein synthesis”. Unfortunately, there are some critical caveats to the conclusions drawn in this study. For example, although the authors generalize by indicating that the effect is on catecholamines, the endogenous levels of different catecholamines were found to be differentially affected by the different inhibitors. In other words, each drug produces different effects on different catecholamines, thus weakening the authors’ conclusion. Moreover, the study failed to provide or explain important factors. For example, it was not tested whether the decreased accumulation of catecholamines following the inhibitors’ treatments was due to the arrest of the synthesis of proteins involved in the metabolism and/or release of catecholamines. All proteins are not translated equally. Some proteins are translated more efficiently than others, thus it is important that specific controls are provided to support the conclusion that the effects on cathecolamine accumulation are caused by actions other than translational inhibition. Consistent with these doubts, the authors (indirectly) recognized that there is no convincing data supporting their conclusion, and, in fact, they stated that: “The mechanisms responsible for the effects of inhibitors of protein synthesis on catecolamines synthesis are unknown”, and “unexplained is the finding that puromycin only affects norepinephrine synthetic rate”. The authors then argue that the side effect hypothesis is nevertheless supported by the correlative finding that the protein synthesis inhibitors often cause transient amnesias, and that memory recovers at 24 hours after treatment, the time at which the catecholamine pool return to normal. However, this, as discussed below, may be a circular argument. Interestingly, coupling between the activation of beta-adrenergic receptors and protein synthesis critical for the late phase of long-term potentiation (L-LTP) has been recently established (Gelinas & Nguyen, 2005). Notably, the activation of beta-adrenergic receptors appears to recruit extracellular signalregulated kinase (ERK) and mammalian target of rapamycin (mTOR) signaling to mediate translation initiation critical for the maintenance of L-LTP (Gelinas, Banko, Hou, Sonenberg, Weeber, Klann, & Nguyen in press, JBC). These findings might explain why similar amnestic properties are seen with both protein synthesis inhibitors and inhibitors of enzymes involved in the metabolism of catecholamines, such as tyrosine hydroxylase and dopamine-beta-hydroxylase (Dismukes & Rake, 1972; Quartermain & Botwinick, 1975; Randt, Quartermain, Goldstein, & Anagnoste, 1971).
Another frequently discussed set of side effects that has been suggested to contribute to memory disruption by protein synthesis inhibitors is the activation of stress activated mitogen-activated protein (MAP) kinases and the superinduction of immediate early genes (IEGs). Results have shown that, for example, anisomycin strongly activates c-Jun NH2-terminal kinase/stress activated protein kinase (JNK/SAPK) and p38/RK in mammalian cells, leading to a rapid induction of IEGs, including c-fos, fosB, c-jun, junB and junD (Cano, Hazzalin, & Mahadevan, 1994; Cano, Doza, Ben-Lev, Cohen, & Mahadevan, 1996). This signaling pathway and the IEG inducing properties of anisomycin were originally believed to be secondary effects of translational arrest that was likely due either to loss of labile repressor proteins or from the stress of translational blockade (Kyriakis, Banerjee, Nicolakaki, Dai, Rubie, Ahmad, Avruch, & Woodgett, 1994; Subramaniam, Schmidt, Crutchfield, & Getz, 1989). However, it was later shown that this is not the case. Anisomycin-stimulated signaling and IEG induction are, in fact, clearly seen at concentrations below those required for translation inhibition (Edwards & Mahadevan, 1992; Mahadevan & Edwards, 1991).
Nevertheless, a number of subsequent, detailed experiments convincingly showed that the superinduction of a set of IEGs is caused selectively by one but not other inhibitors (Hazzalin, Le Panse, Cano, & Mahadevan, 1998), leading, once again, to the conclusion that that although protein synthesis inhibitors can exert side effects, these appear to be drug-specific. In fact, different gradients of transcript superinduction are produced by different inhibitors, and each inhibitor targets different mechanisms. Here, I will cite only some illustrative examples among several provided in the literature (e.g. Cano et al., 1994; Sidhu & Omiecinski, 1998). When administered with epidermal growth factor (EGF), anisomycin, and to a much lesser extent cycloheximide, result in rapid phosphorylation of the kinases pp33 and pp15, but only anisomycin, and not cycloheximide, activates p45 and p55. Moreover, both p45 and p55 kinases are not activated by either emetine or puromycin (Cano et al., 1994). Similarly, another study reported that anisomycin can activate stress-activated protein kinases, MAP kinase and other signal transduction pathways, such as JNK/SAPK (c-Jun NH2-terminal kinase/stress activated protein kinases) and p38/RK resulting in rapid induction of immediate-early genes. This response is however almost absent or not found at all with other protein synthesis inhibitors, including cycloheximide, emetine and puromycin. This study also showed that anisomycin acts like a true signaling agonist in eliciting highly specific homologous desensitization, but that, again, this effect is selective for this protein synthesis inhibitor and is not elicited by the others (Hazzalin et al., 1998). In fact, these authors stated that: “…The signaling and IEG-inducing properties of anisomycin were originally thought to be secondary effects of translational arrest, arising either from the loss of labile repressive proteins (55) or from the stress of translational arrest (34). However, the fact that anisomycin-stimulated signaling and gene responses are clearly demonstrable at concentrations below those required for inhibiting translation, and, conversely, not all translational inhibitors activate signaling responses. Puromycin and emetine have negligible signaling and gene-inducing effects and, although cycloheximide has some ability to activate these signaling responses, it is very much weaker than that of anisomycin, whereas it blocks translation equally well” (Hazzalin et al‥ 1998). In summary, although anisomycin treatment can elicit a number of molecular activations, they seem to be specific for this compound and not shared with other protein synthesis inhibitors.
Finally, apoptosis has also been considered as a potential side effect of protein synthesis inhibitors employed in learning and memory studies (Rudy et al., 2006).
In fact, protein synthesis inhibitors have been reported to have contrasting effects on cell survival: they can either cause apoptosis or protect from apoptosis (e.g. Martin, Green, & Cotter, 1994; Rehen, Varella, Freitas, & Moraes, 1996; Tessitore, Tomasi, & Greco, 1999; Torocsik & Szeberenyi, 2000). To my knowledge, none of the studies on memory consolidation or reconsolidation have conducted parallel cellular and/or molecular investigations testing any of these two opposing outcomes in brain preparations. In cell lines or other biological preparations, the two opposing outcomes have been found to vary with the type of inhibitor used, the concentration and the experimental conditions. Moreover, it has not yet been fully understood whether the apoptotic effects are due to inhibition of protein synthesis (Cano et al., 1994; Chow, Peters, & Orrenius, 1995). Interestingly, there seems to be an important link between translational regulation and apopotosis (Clemens, 2001). Thus, whether apoptosis occurs in the central nervous system following the administration of protein synthesis inhibitors during or in proximity to learning or memory reactivation and contributes to the amnesia remains to be proven and should be tested in each conditions and dosage employed. Nevertheless, although several questions concerning the relationship between apoptosis and protein synthesis still remain to be addressed, control experiments showing that general toxicity due to cell death did not contribute to amnesia caused by protein synthesis inhibitors have been provided in several learning and memory paradigms. These control experiments showed that, although the protein synthesis inhibitors resulted in amnesia, they did not prevent further learning (Duvarci & Nader, 2004; Milekic & Alberini, unpublished), indicating that amnesia was not due to cell disruption or general cell death.
In summary, the literature seems to reveal the following conclusions: first, the mechanism of action of each of the inhibitors commonly used to study learning and memory is different, as each affects translation by targeting distinct mechanisms. Second, the side effects of protein synthesis inhibitors appear to be either secondary to protein synthesis arrest or are not common to all translation inhibitors, but rather specific for each compound. Indeed, side effects common to all the different protein synthesis inhibitors still remain to be identified. Third, the studies arguing for a contribution of side effects toward amnesia do not provide formal controls to exclude that the side effects under investigation is imputable to translation inhibition of specific proteins.
Fourth, an additional important finding concerns the consistent temporal boundaries of the amnestic effect of protein synthesis inhibitors on memory consolidation and reconsolidation. If the inhibitors are administered sometime after (e.g. few hours) either training or memory reactivation, memory retention is not affected. If side effects were the main cause of amnesia, we would need to postulate the unlikely hypothesis that training and memory reactivation set in motion changes in the brain that respond toward a variety of different side effects exerted by different compounds only during a common, limited temporal window.
Thus, although it is possible that some results in learning and memory or synaptic plasticity studies are imputable to side effects of the specific inhibitor utilized, a formal demonstration that the amnesia produced by protein synthesis inhibitor administration is due to side effects of these compounds seems to be lacking. Conversely, there are several hundred reports which employed several different inhibitors, including cycloheximide, acetoxycycloheximide, anisomycin, puromycin and emetine documenting that long-term memory and long-term synaptic plasticity are impaired after treatment. Finally, only a handful of reports showed that some long-term memory is not affected by protein synthesis inhibition. Thus, all this evidence seems to be consistent with the conclusion that protein synthesis is required for long-term memory and long-term synaptic plasticity.
It has been suggested that one other result that refutes the critical role of protein synthesis in long-term memory formation is that modification of the training procedures such as use of stimulants, increasing foot shock intensity, providing a reminder footshock or pre-training manipulations can eliminate the requirement for protein synthesis and turn memory formation into a protein synthesis-independent process (Routtenberg & Rekart, 2005). This view, however, neglects the fact that modifications to the training protocol, such as those mentioned above, are themselves additional learning experiences, and, as such, are very likely to set in motion changes in protein synthesis (e.g. Ronnback & Hansson, 1986; Smith, Starch, Roberts, & Schuman, 2005). This supplementary protein synthesis may be able to compensate and/or add to in both time and/or space (anatomical distribution) to that induced by the original training. Similarly, stimulants hormones and neurotransmitters including amphetamine, caffeine, nicotine, and corticosterone are known to induce gene expression (e.g. Gonzalez-Nicolini & McGinty, 2002; Li, Kane, Wang, & Ma, 2004; Morsink, Steenbergen, Vos, Karst, Joels, De Klowet, & Datson, 2006; Sokolov, Polesskaya, & Uhl, 2003). In other words, it would not be surprising that a quantum of protein synthesis inhibition following an original protocol may not sufficiently disrupt also the protein synthesis induced by additional behaviors or pharmacological treatments. Additionally, this quantum could be overcome by stronger training protocols, additional pharmacological stimulation that might lead to a protein synthesis phase or phases (Grecksch & Matthies, 1980; Freeman, Rose, & Scholey, 1995; Quevedo, Vianna, Roesler, de-Paris, Izquierdo, & Rose, 1999; Quevedo, Vianna, Roesler, Martins, de-Paris, Medina, & Izquierdo, 2005) whose duration may exceed the blocking effect of one dose of protein synthesis inhibitor.
3. The role of protein synthesis in long-term synaptic plasticity and memory: evidence from molecular-targeting approaches
Notably, other molecular approaches have confirmed that the synthesis of proteins is an essential molecular step of memory formation. One strategy utilized to selectively block the synthesis of specific proteins is the delivery of antisense sequences or the expression of dominant negative molecules (e.g. Alberini, Ghirardi, Metz, & Kandel, 1994; Cheli, Adrover, Blanco, Rial Verde, Guyot-Revol, Vidal, Martin, Alche, Zanchez, Acerbo, Epstein, & Jerusalinsky, 2002; Garcia-Osta, Tsokas, Pollonini, Landau, Blitzer, & Alberini, 2006; Guzowski & McGaugh, 1997; Lee, Everitt, & Thomas, 2004; Johnson & Rose, 2001; Taubenfeld, Milekic, Monti, & Alberini, 2001b; Tronel, Milekic, & Alberini, 2005; Milekic. Pollonini, & Alberini, in press; Yin, Wallach, Del Vecchio, Wilder, Zhou, Quinn, & Tully, 1994). The antisense as well as small interfering RNA (siRNA) methods inhibit the translation of specifically targeted mRNAs. Moreover, in the brain, these strategies allow the investigator to target gene regulation in an anatomically and temporally restricted fashion. The antisense technology has been studied for almost 30 years. The mechanisms of action, stability, specificity and delivery issues have been largely investigated and a great deal is now known about the use of antisense sequences in vivo (Estibeiro & Godfray, 2001; Forte, Cipollaro, Cascino, & Galderisi, 2005; Trulzsch & Wood, 2004). The effect of the antisense treatment compared to relative controls, including sequences containing the same base composition but in scrambled order which are not complementary to any known RNA sequences, indicates that the ongoing expression of the corresponding protein is critical for the function tested (e.g. memory). More than 100 papers have reported significant effects of antisense sequences during memory consolidation, memory reconsolidation, long-term synaptic plasticity or brain long-term changes in general. Among the proteins targeted, there are transcription factors such as CREB, C/EBP, zif 268, fos, nur 77, neurotransmitter receptors, such as NR1 and alpha7 acethylcholine receptors, growth factors such as BDNF and many other molecules including arc, APP and SNAP-25. Routtenberg and Rekart (2005) challenge the conclusion that the synthesis of specific molecules is required for memory formation, and suggest that the effect of antisense sequences on memory retention might be related to a consequent rebound from the antisense-induced inhibition of protein expression, which in some case has been documented (Guzowski & McGaugh, 1997). To my knowledge, the rebound was described only in this one case. Nevertheless, if this explanation is true, it would imply that the translation during the rebound phase is necessary for memory formation. Hence, although in a more indirect fashion, such outcome would still support the critical role of protein translation during memory formation. Furthermore, results similar to those obtained with antisense sequences have been reported using several other approaches including temporally-regulated expression of dominant negative molecules (Chen, Muzzio, Malleret, Bartsch, Verbitsky, Pavlidis, Yonan, Vronskaya, Grody, Cepeda, Gilliam, & Kandel, 2003; Yin et al., 1994), interfering decoys (e.g. Alberini et al., 1994; Athos, Impey, Pineda, Chen, & Storm, 2002; Dash, Hochner, & Kandel, 1990; Dash, Orsi, & Moore, 2005; Freudenthal, Boccia, Acosta, Blake, Merlo, Baratti, & Romano, 2005) or blocking antibodies (Alberini et al., 1994; Bartsch, Casaio, Karl, Serodio, & Kandel, 1998). Given the consistency of the findings with different molecular blocking approaches and the high number of different molecules targeted, it is reasonable to conclude that it is the blocking effect that unravels the biosynthestic molecular requirements for memory formation.
Additional support to this conclusion is offered by the observations that, in numerous studies, changes in the profiles of gene and protein expression has been documented (Azami, Wagatsuma, Sadamoto, Hatakeyama, Usami, Fujie, Koyanagi, Azumi, Fujito, Lukowiak, & Ito, 2006; D'Agata & Cavallaro 2002; Leil, TA, Ossadtchi, Nichols, Leahy, & Smith, 2003; McNair, Broad, Riedel, Davies, & Cobb, 2007; O'Sullivan, McGettigan, Sheridan, Pickering, Conboy, O'Connor, Moynagh, Higgins, Regan, & Murphy, 2007; Park, Onodera, Nishimura, Thompson, & Itohara, 2006) and the functional requirement of specific proteins during long-term memory or long-term synaptic plasticity formation is accompanied by a transient increase in their mRNA and/or protein expression. This has been particularly evident and characterized in the case of regulatory IEGs. Such an outcome, precisely because it concerns inducible transcription factors, whose function is to regulate the expression of target genes, strengthen the conclusion that phases of increased gene expression are coupled to learning events (Alberini et al., 1994). For example, using a decoy oligodeoxynucleotide that interferes with its DNA binding activity, we have shown that in the invertebrate Aplysia californica, the transcription factor CCAAT enhancer binding protein (C/EBP) is required for up to 9, but not 12 hours after the induction of long-term facilitation (Fig. 1) (Alberini et al., 1994). Importantly, the requirement for C/EBP during Aplysia long-term facilitation was also confirmed using two additional approaches: antisense-mediated knock-down and antibodies that were shown to functionally block the DNA binding activity of C/EBP (Alberini et al., 1994). Moreover, in rat hippocampus, we found that C/EBPbeta is induced starting at 9 hours after inhibitory avoidance training, remains increased at 20 and 28 hours after training and returns to control levels at 48 hours after training (Fig. 2) (Taubenfeld, Wiig, Monti, Dolan, Pollonini, & Alberini, 2001a). The functional requirement of hippocampal C/EBPbeta parallels its profile of learning-related expression increase, as in fact antisense-mediated knock-down of C/EBPbeta in the hippocampi blocks memory consolidation at 5 and 24 hours after training but not 1 hour before or 46 hours after training (Fig. 3) (Taubenfeld et al., 2001b). These results frame the temporally-limited expression regulation and functional requirement of hippocampal C/EBPbeta during the consolidation phase of memory, suggesting that a cascade of gene expression is initiated by learning and remains active for more than 24 hours after training. This cascade of molecular events is therefore a critical molecular step for the establishment of long-lasting memories.
Figure 1.
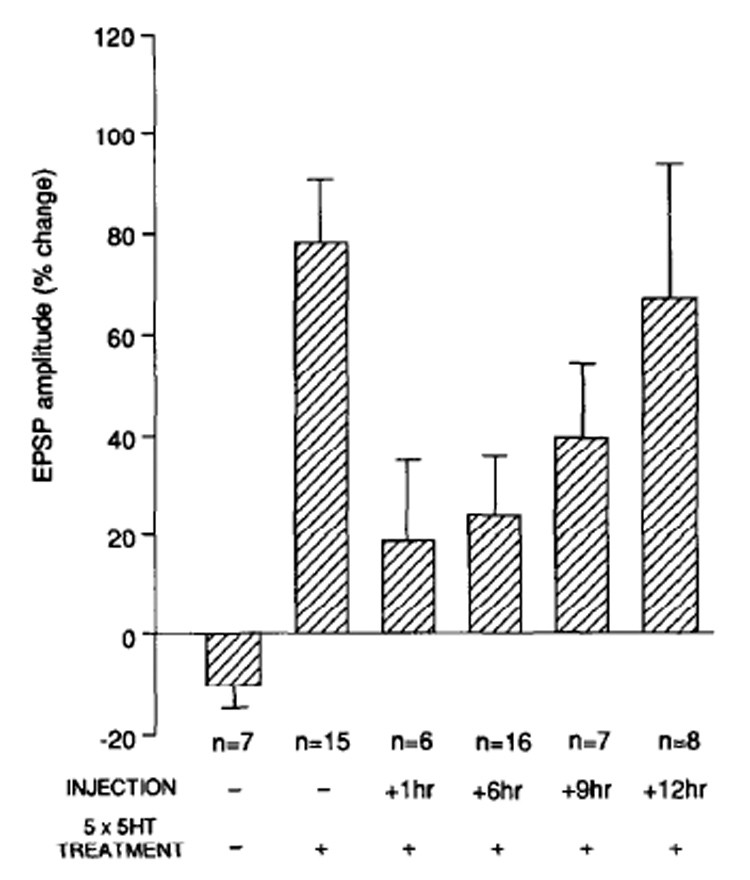
Time course of the effect of the ERE decoy following 5-HT treatment. Bar graph representing the percentage of change ±b SEM in EPSP amplitude recorded 24 hr after 5 pulses of 5-HT from cocultures injected with the C/EBP binding site ERE oligonucleotide at the indicated times after the end of 5-HT applications (From Alberini et al., 1994).
Figure 2.
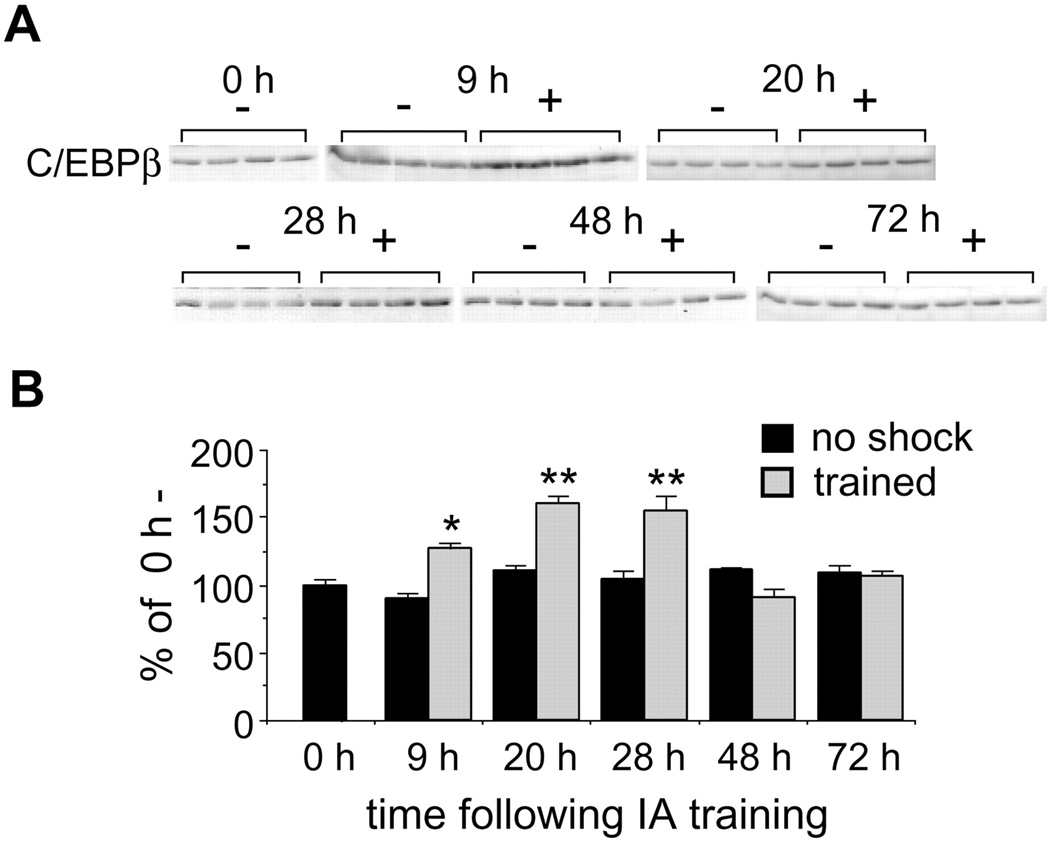
C/EBPbeta protein is selectively induced after inhibitory avoidance training. Western blot analyses were performed with anti-C/EBP antiserum. A, Western blot immunostaining of hippocampal extracts from rats killed immediately (0 h) and 9, 20, 28, 48, and 72 hr after training. Groups of animals either (1) underwent full training (+) or (2) entered the IA apparatus but received no shock (−). B, Densitometric analysis of C/EBPbeta Western blot depicted in A revealed a significant increase in C/EBPbeta protein at 9 hr (p < 0.05), 20 hr (p < 0.01), and 28 hr (p < 0.01) compared with 0 h- control rats. There were no significant differences in any of the time-paired no shock control groups. Data are expressed as mean percentage ± SEM of the 0 h (100%) control mean values (From Taubenfeld et al., 2001a).
Figure 3.
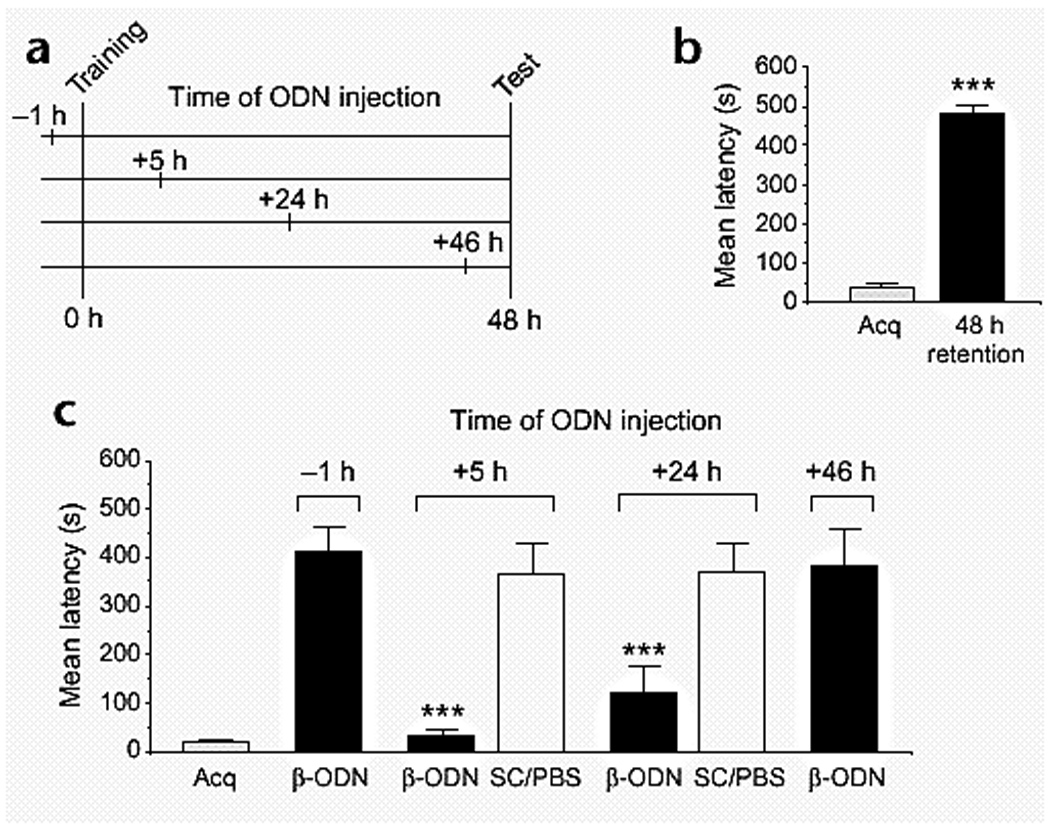
C/EBPbeta antisense blocks long-term memory consolidation. (a) Injection, training and testing time points. (b) IA acquisition (Acq) and memory retention of unoperated rats. Retention was assessed 48 h after training by measuring the latency to reenter a context previously paired with a foot-shock. A single training event leads to a significant increase in latency (n = 16, *** p < 0.0001). (c) Hippocampal injection of C/EBPbeta antisense (-ODN) 5 h (n = 12) or 24 h (n = 8) after IA training significantly blocks memory retention at 48 h compared to SC-ODN-injected or unoperated groups (*** p < 0.0001). Injection of SC-ODN sequence or vehicle (PBS) at same time points has no effect. Because both controls produced similar retention latencies, the behavioral data of the two groups have been combined (SC/PBS) (5 h, n = 9, SC-ODN, n = 5 and PBS, n = 4; 24 h, n = 8, SC-ODN, n = 4 and PBS, n = 4). Injection of -ODN 1 h before (n = 9) or 46 h after (n = 7) training does not produce any significant memory impairment (From Taubenfeld et al., 2001b).
Another set of evidence that also supports the conclusion that protein synthesis is critical for memory formation is the discovery that intact functional components of the translational machinery are critical for long-term memory and synaptic plasticity (Banko, Hou, Polin, Sonenberg, & Klann, 2006; Costa-Mattioli, Gobert, Harding, Herdy, Azzi, Bruno, Bidinosti, Ben Mamou, Marcinkiewicz, & Yoshida, 2005; Kelleher, Govindarajan, Jung, Kang, & Tonegawa, 2004; Richter, 2007; Tirosh, Elkobi, Rosenblum, & Meiri, 2007). For example, a recent study show that phosphorylation-dependent regulation of the Initiation Factor 2 α (eIF2α) is a critical hub for the control of synaptic plasticity and memory. eIF2α is an initiation factor of translation, thus it controls the overall rate of protein synthesis through its effects on the translation machinery. In addition to this general effect, eIF2α also controls the rate of translation of specific proteins, including that of the transcriptional repressor ATF4, an antagonist of CREB-mediated gene transcription. Costa-Mattioli, Gobert, Stern, Gamache, Colina, Cuello, Sossin, Kaufman, Pelletier, Rosenblum, Krnjevic, Lacaille, Nader, and Sonenberg (2007) recently reported that hippocampal synaptic plasticity, associative fear conditioning, spatial learning and memory, and novel taste memory require the function of eIF2a, which is regulated through its phosphorylation. Specifically, they demonstrated that eIF2α dephosphorylation at serine 51 is associated with augmented memory for spatial navigation, Pavlovian fear conditioning and conditioned taste aversion. These results underline the importance of activity-induced protein translation during long-term synaptic plasticity and memory.
Thus, it is consistent with a multitude of controlled experiments to conclude that protein synthesis is required for long-term synaptic plasticity and memory formation. In my view, the question that remains to be addressed is not if but how protein synthesis allows long-term memory and plasticity to occur. The relevant questions are numerous: Is synaptic vs cell body protein synthesis more or less critical? What is the temporal profile of the critical phase/s of protein synthesis? Does it target specific neurons and synapses and how? What is the role of this protein synthesis? Does it refill the homeostatic pool at activated synapses? Does modulation contribute to the protein synthesis-dependent changes of memory consolidation? All these very important questions are under investigation (e.g. Gold, 2006; Klann & Dever, 2004; Martin & Zukin, 2006; Milekic & Alberini, 2002; Routtenberg & Rekart, 2005; Sutton & Schuman, 2006) and beyond the scope of this review, and likely to be addressed in the near future.
4. Requirement for protein synthesis during memory formation: temporary or persistent effect?
Because inhibitors of protein synthesis in some cases have caused only temporary amnesia, and the memory can spontaneously recovers after some time, it has been questioned whether the effect of these treatments targets memory retrieval rather than consolidation or reconsolidation processes (e.g. Anokhin, Tiunova, & Rose, 2002; Lattal & Abel, 2004; Power, Berlau, McGaugh, & Steward, 2006; Riccio, Moody, & Millin, 2002; Riccio. Millin, & Bogart, 2006; Salinska, Bourne, & Rose, 2004). However, opposite results have been reported in other cases, in which the memory deficits observed after the administration of protein synthesis inhibitors were persistent (e.g. Child, Epstein, & Kuzirian, 2003; Debiec, LeDouz, & Nader, 2002; Lattal & Abel, 2004; Suzuki, Josselyn, & Frankland, 2004), and, furthermore, the experience of a reminder failed to restore memory retention (e.g. Anokhin et al., 2002; Boccia, Blake, & Acosta, 2005; Davis & Rosenzweig, 1978; Duvarci & Nader, 2004). The reader should refer to an excellent, exhaustive and updated discussion of this matter by Larry Squire (2007). Here, I will comment on some additional evidence that can explain the opposite outcomes on the duration of amnesia after translation inhibition.
One aspect that is unfortunately often neglected in experiments using protein synthesis inhibitors in learning and memory is the parallel accurate assessment of the degree and duration of protein synthesis inhibition. Very few authors studying memory or plasticity have also reported the rate of protein synthesis inhibition in their experiments, and often use references describing inhibition of protein synthesis in different brain areas and under different experimental conditions as general principles. However, it is critical that, as detailed above, the effect of protein synthesis inhibitors be assessed in each experimental model, as variations of protein synthesis occur in relation to dosage, route of administration, brain areas, behavioral protocols, etc.
Studies carried out in the 1970s proved that stronger memories require longer (stronger) inhibition of protein synthesis to be permanently disrupted, suggesting that, in fact, a partial inhibition may exactly be the reason why a transient amnesia can be seen (Barraco & Stettner, 1976; Davis & Rosenzweig, 1978; Flood, Bennett, Orme, & Rosenzweig, 1975; Flood, Rosenzweig, Bennett, & Orme, 1973).
In agreement, a recent work from my laboratory has demonstrated that a partial inhibition of protein synthesis during the consolidation or reconsolidation phase precisely results in temporary behavioral impairments that recover at later times. However, a more prolonged inhibition led to a persistent disruption of the behavioral response (Milekic, Brown, Castellini, & Alberini, 2006). In this study, we assessed the effect of two widely used protein synthesis inhibitors, anisomycin and cycloheximide on the formation of conditioned place preference to morphine (mCPP). The two inhibitors, which block protein synthesis by distinct mechanisms, were used in order to confirm that the results were specifically related to protein synthesis inhibition and not to unspecific effects. Both inhibitors produced similar outcomes. Rats were conditioned to morphine or vehicle once a day for four days, and at the end of a four-day conditioning session, half of the morphine-conditioned rats received a single peripheral injection of either anisomycin or cycloheximide while the other half received vehicle solution. CPP was tested 24 hours later. Both inhibitors significantly blocked mCPP. To determine whether the effect was stable, the animals were retested 1 week later. At this time, the animals showed a partial, but significant recovery of the place preference, suggesting that that inhibition of protein synthesis at the end of conditioning impaired CPP only transiently (Fig. 4A).
Figure 4.
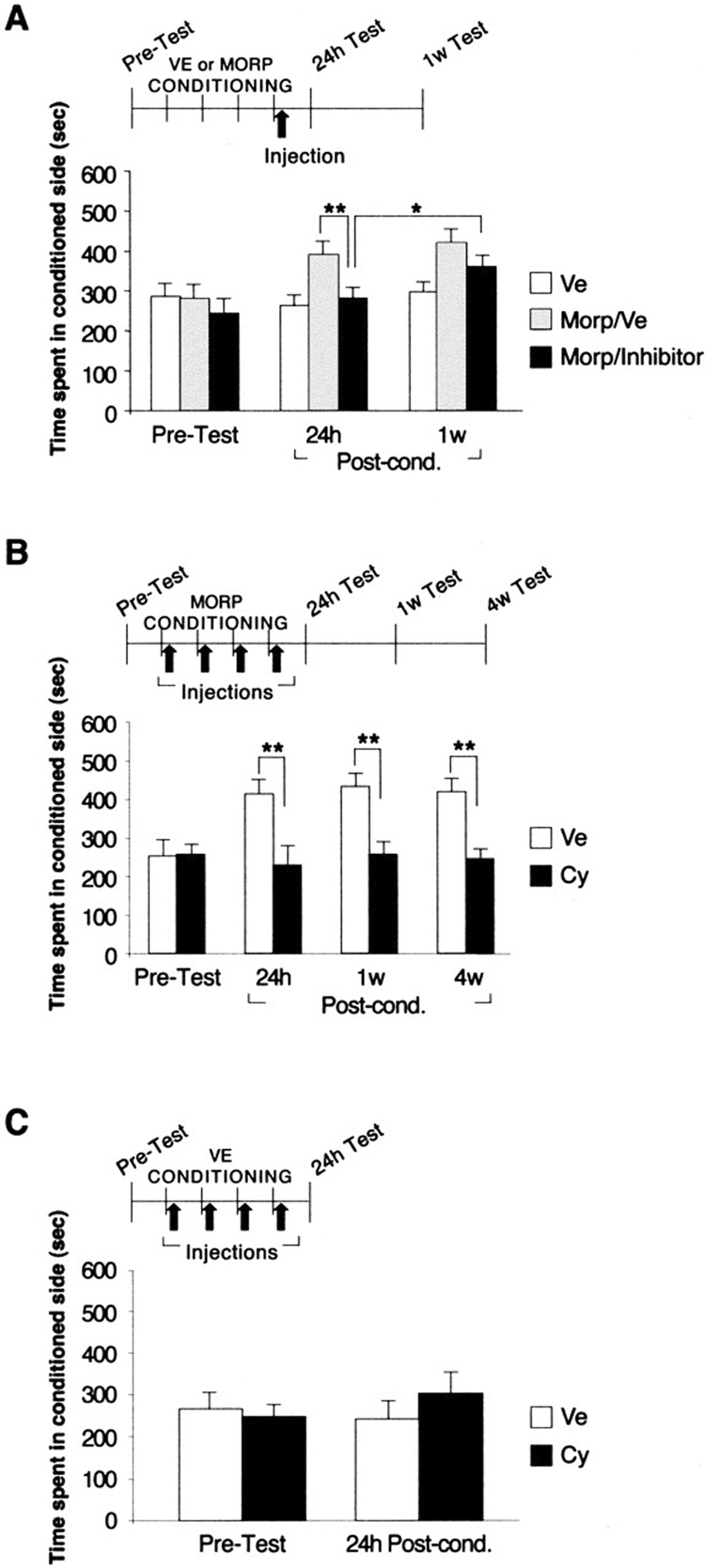
Protein synthesis is required for the induction of mCPP. A–C, Values are expressed as mean ± SEM time spent in the drug-conditioned chamber. *p < 0.05; **p < 0.01. A, Groups of rats were conditioned for 4 d to either vehicle (n = 8; white bars) or morphine (n = 15). At the end of conditioning, the morphine-conditioned rats were injected subcutaneously with either vehicle (n = 8; gray bars) or inhibitor (n = 7, of which n = 4 with cycloheximide and n = 3 with anisomycin; black bars). All rats were tested 24 h and 1 week later. B, Rats received daily administration of either cycloheximide (n = 8; black bars) or vehicle (n = 8; white bars) immediately after each conditioning session. CPP was tested 24 h, 1 week, and 4 weeks later. C, Rats were conditioned to vehicle instead of morphine and administered cycloheximide (n = 9; black bars) or vehicle (n = 11; white bars) immediately after each conditioning session. CPP was tested after 24 h. VE or Ve, Vehicle; MORP or morp, morphine; w, week; Post-cond., postconditioning; Cy, cycloheximide (From Milekic et al., 2006).
We therefore investigated whether, as suggested by the results of Flood et al. (1975), a partial inhibition of protein synthesis delayed, but did not sufficiently disrupt the consolidation process. Consistent with this hypothesis, we also considered the possibility that each conditioning event induces a protein synthesis-dependent phase and that blocking protein synthesis only during the last day of conditioning is not sufficient to disrupt the consolidation of mCPP.
We first tested whether a more extended inhibition of protein synthesis at the end of the 4-day conditioning results in a persistent blockade of mCPP. We injected the animals twice with cycloheximide, the first time immediately after and the second time 5 hours after the last conditioning session. In parallel, the rates of protein synthesis inhibition, over time, following a single or double injection were established, by measuring the incorporation of 35S methionine. Similar to that described by Flood et al. (Flood, Bennett, Orme, & Rosenzweig, 1978; Flood, Jarvik, Bennett, Orme, & Rosenzweig, 1977), we found that at one hour after a single injection, 70% of protein synthesis was inhibited, while at 6 hours, only 23% of protein synthesis was blocked. With the double injection, the rate of protein synthesis inhibition at 6 hours was maintained at 71%. These results confirm that single doses of protein synthesis inhibitors administered systemically significantly block protein synthesis only for a very short time (in our case approximately 1–3 hours). Thus, it is not surprising that several similar experiments based on the use of only one dose of inhibitor can cause transient amnesia when employed in learning paradigms that induce memories lasting for weeks or months.
When we applied the double injection protocol, which led to a more extended inhibition of protein synthesis at the end of conditioning, the behavioral results were similar to those found after a single injection. CPP was significantly inhibited at 24 hours but recovered one week later. However, a very different result emerged when rats received one dose of either cycloheximide or vehicle every day at the end of each conditioning trial. The animals were completely impaired in mCPP at 24 hours, and the impairment was maintained at 1 and 4 weeks after the end of treatment (Fig. 4B). Thus, blocking protein synthesis after each conditioning session persistently disrupts the consolidation of mCPP.
We also found similar results when we investigated the effect of protein synthesis inhibitors on the reactivation of mCPP, which was accomplished by the re-experience of a single reinforced trial, i.e. a conditioning session. This reactivation trial was administered 1 week after the end of the initial 4-day conditioning period. The systemic administration of either cycloheximide or anisomycin caused a significant disruption of mCPP 24 hours later. However, 1 week later, the disruption did not persist (Fig.5A). Subsequent experiments tested the effect of two injections of cycloheximide, one immediately after the re-experience of a conditioning session and the second 5 hours later. This treatment was able to persistently block mCPP (Fig. 5B). Several control tests excluded that the disruption of mCPP by cycloheximide was due to an avoidance of the conditioning context induced, for example, by malaise caused by the treatment itself (Fig.5C). Moreover, the blocking effect of the inhibitors on mCPP following reactivation by conditioning was found to be context-dependent, because when the conditioning context was omitted and reactivation was experienced in a different context, no effect was found (Fig.5D) (Milekic et al., 2006). Lesions or cell injury or death of brain cells was generally excluded because the rats in which CPP was disrupted were successfully conditioned following a new 4-day conditioning (data unpublished).
Figure 5.
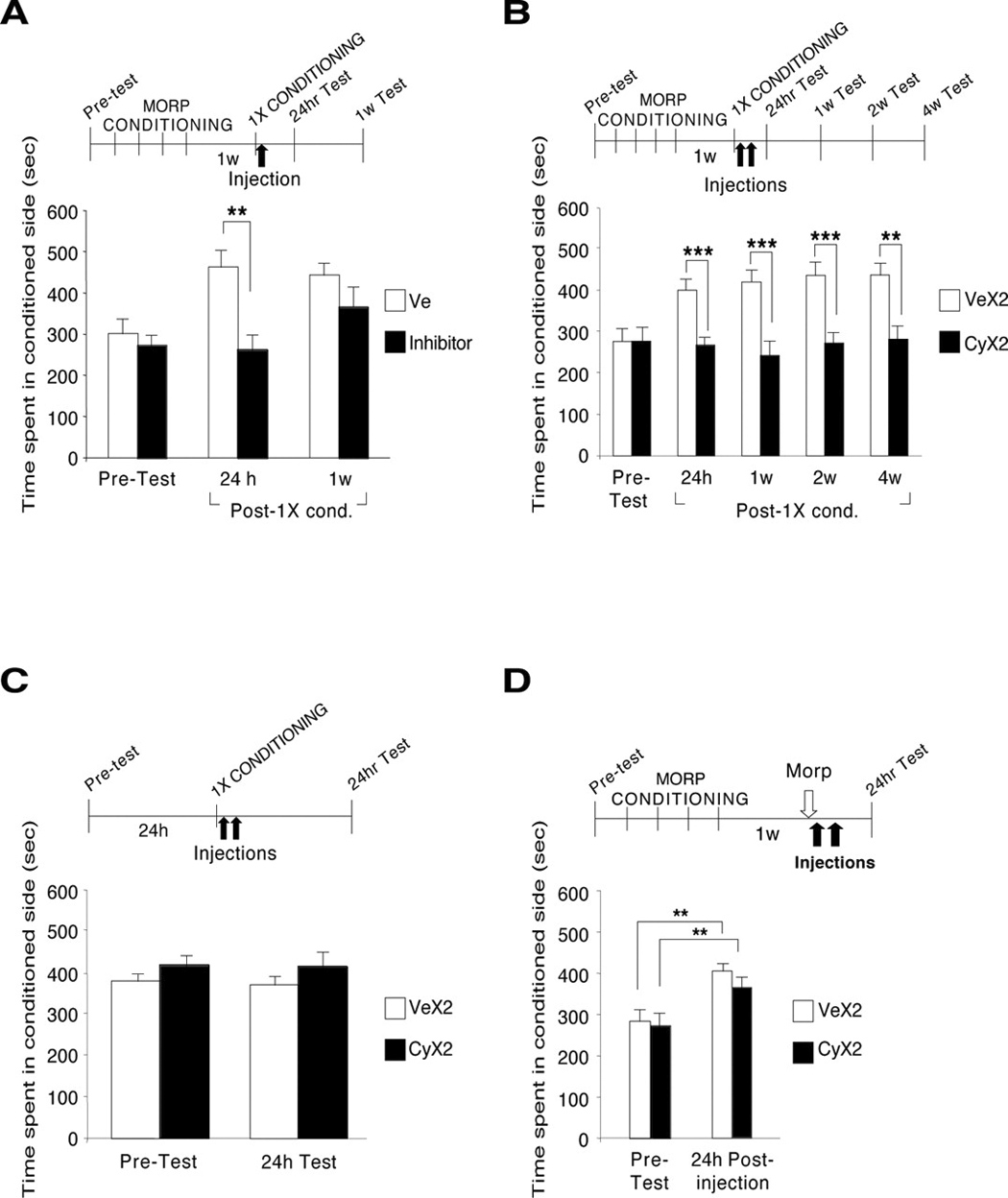
An established mCPP is disrupted by protein synthesis inhibitors administered after a single conditioning session. A–D, Values are expressed as mean ± SEM time spent in the drug-conditioned chamber. **p < 0.01; ***p < 0.001. A, Rats were conditioned to morphine and 1 week later received one additional conditioning session (1X CONDITIONING). Immediately after, one-half of the animals were injected with cycloheximide or anisomycin (inhibitors; n = 10, of which n = 6 with cycloheximide and n = 4 with anisomycin; black bars), and the other half were injected with vehicle (n = 8; white bars). Rats were tested 24 h and 1 week later (Post-1X cond.). B, Animals were conditioned and 1 week later received one additional conditioning (1X CONDITIONING) as described in A, followed by two injections of cycloheximide (CyX2; n = 10; n = 7 in 4 week test; black bars) or vehicle (VeX2; n = 11; white bars) 5 h apart. Rats were tested 24 h and 1, 2, and 4 weeks later. C, Rats received one morphine conditioning in the spontaneously preferred context followed by two injections of either cycloheximide (CyX2; black bars; n = 8) or vehicle (VeX2; white bars; n = 8) and were tested 24 h later. D, The conditioning context was omitted. Rats were conditioned for 4 d and 1 week later received one morphine treatment in their home cage. Thirty minutes later, they received two injections of either cycloheximide (CyX2; black bars; n = 13) or vehicle (VeX2; white bars; n = 12). CPP was tested 24 h after injection. MORP, Morphine; w, week; Ve, vehicle (From Milekic et al., 2006).
Thus, our results show that mCPP can be persistently disrupted by inhibiting protein synthesis either immediately following each conditioning event or after the concomitant reactivation of both the contextual cues and the experience of the drug. The persistence of the effect requires, however, that protein synthesis be sufficiently disrupted.
By definition, it is always impossible to exclude that amnesia results from a retrieval deficit, because even though no recovery might be observed under any circumstances, there is no direct method yet available that can prove that the memory trace has been completely erased. However, our data corroborate the conclusion that, in many cases, a recovery of memory retention after some time might be due to an insufficient blockade of the protein synthesis phase elicited by training or memory reactivation. In general, the same principles apply to both consolidation and reconsolidation studies. However, as memory reconsolidation is less well understood, additional questions need to be addressed in relation to the type of proteins involved, the temporal duration and the rate of protein synthesis required (Alberini, 2005; Alberini, 2007; Dudai, 2004; Gold, 2006; Lattal & Abel 2004; Miller & Sweatt 2006; Rudy et al., 2006). Importantly, the effect of protein synthesis inhibition after memory reactivation is likely to be more complex, and, in fact, it needs to take into account other aspects that involve additional phases of protein synthesis, such as the fact that consolidation has in part already occurred and the possible additional implicit nature of reactivation experiences (Anokhin et al., 2002; Alberini, 2007).
5. Conclusions
Numerous studies provide strong evidence that the expression of specific proteins during an early and temporally limited phase after learning is necessary for the stabilization or consolidation of long-term memory and synaptic plasticity. Although this conclusion is still sometimes challenged with arguments of potential non-specific effects, a number of different approaches that include the use of a variety of inhibitors of protein synthesis, antisense sequences, regulated expression of dominant negative molecules, blocking antibodies, identification of gene expression profiles and detailed investigations of the mechanisms of translation activated by learning or memory reactivation, all seem to converge on the concept that protein synthesis plays an essential role in the stabilization of both new and reactivated memories. The functional processes that this protein synthesis subserves during memory consolidation and storage still remain to be understood, and their understanding is the object of existing investigations.
Acknowledgements
many thanks to Bob Blitzer, Deanna Benson for their helpful comments. Thanks to all the members of my lab for their invaluable contribution to the work discussed and for their helpful feedbacks on the manuscript and to Dr. Reginald Miller and the CCMS facility of Mount Sinai for technical support. The work included in this review was supported by the National Institute of Mental Health (R01 MH65635, R01 MH074736), National Institute of Drugs of Abuse (R21 CEBRA DA017672) and Hirschl Foundation to CMA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends in Neuroscience. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Reconsolidation: The Samsara of Memory Consolidation. Debates in Neuroscience. 2007;1:17–24. [Google Scholar]
- Anokhin KV, Tiunova AA, Rose SP. Reminder effects - reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. European Journal of Neuroscience. 2002;15:1759–1765. doi: 10.1046/j.1460-9568.2002.02023.x. [DOI] [PubMed] [Google Scholar]
- Athos J, Impey S, Pineda VV, Chen X, Storm DR. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nature Neuroscience. 2002;5:1119–1120. doi: 10.1038/nn951. [DOI] [PubMed] [Google Scholar]
- Azami S, Wagatsuma A, Sadamoto H, Hatakeyama D, Usami T, Fujie M, Koyanagi R, Azumi K, Fujito Y, Lukowiak K, Ito E. Altered gene activity correlated with long-term memory formation of conditioned taste aversion in Lymnaea. Journal of Neuroscience Research. 2006;84:1610–1620. doi: 10.1002/jnr.21045. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proceedings of the National Academy of Sciences USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko L, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. Journal of Neuroscience. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M, Vázquez D. Ribosome changes during translation. Journal of Molecular Biology. 1975;93:449–463. doi: 10.1016/0022-2836(75)90239-9. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- Barraco RA, Stettner LJ. Antibiotics and memory. Psychological Bulletin. 1976;83:242–302. [PubMed] [Google Scholar]
- Bennett LL, Smithers D, Ward CT. Inhibition of DNA synthesis in mammalian cells by actidione. Biochimica et Biophysica Acta. 1964;87:60–69. doi: 10.1016/0926-6550(64)90047-7. [DOI] [PubMed] [Google Scholar]
- Boccia MM, Blake MG, Acosta GB. Memory consolidation and reconsolidation of an inhibitory avoidance task in mice: effects of a new different learning task. Neuroscience. 2005;135:19–29. doi: 10.1016/j.neuroscience.2005.04.068. [DOI] [PubMed] [Google Scholar]
- Cano E, Doza YN, Ben-Levy R, Cohen P, Mahadevan LC. Identification of anisomycin-activated kinases p45 and p55 in murine cells as MAPKAP kinase-2. Oncogene. 1996;12:805–812. [PubMed] [Google Scholar]
- Cano E, Hazzalin CA, Mahadevan LC. Anisomycin-activated protein kinases p45 and p55 but not mitogen-activated protein kinases ERK-1 and -2 are implicated in the induction of c-fos and c-jun. Molecular Cell Biology. 1994;14:7352–7362. doi: 10.1128/mcb.14.11.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli VT, Adrover MF, Blanco C, Rial Verde E, Guyot-Revol V, Vidal R, Martin E, Alche L, Sanchez G, Acerbo M, Epstein AL, Jerusalinsky D. Gene transfer of NMDAR1 subunit sequences to the rat CNS using herpes simplex virus vectors interfered with habituation. Cellular and Molecular Neurobiology. 2002;22:303–314. doi: 10.1023/A:1020720001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, Yonan AL, Vronskaya S, Grody MB, Cepeda I, Gilliam TC, Kandel ER. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–569. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Child FM, Epstein HT, Kuzirian AM. Memory reconsolidation in hermissenda. The Biological Bulletin. 2003;205:218–219. doi: 10.2307/1543261. [DOI] [PubMed] [Google Scholar]
- Chow SC, Peters I, Orrenius S. Reevaluation of the role of de novo protein synthesis in rat thymocyte apoptosis. Experimental Cell Research. 1995;216:149–159. doi: 10.1006/excr.1995.1019. [DOI] [PubMed] [Google Scholar]
- Clemens MJ. Translational regulation in cell stress and apoptosis. Roles of the eIF4E binding proteins. Journal of Cellular and Molecular Medicine. 2001;5:221–239. doi: 10.1111/j.1582-4934.2001.tb00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjevic K, Lacaille JC, Nader K, Sonenberg N. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agata V, Cavallaro S. Gene expression profiles--a new dynamic and functional dimension to the exploration of learning and memory. Reviews in the Neurosciences. 2002;13:209–219. doi: 10.1515/revneuro.2002.13.3.209. [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Sequestration of serum response factor in the hippocampus impairs long-term spatial memory. Journal of Neurochemistry. 2005;93:269–278. doi: 10.1111/j.1471-4159.2004.03016.x. [DOI] [PubMed] [Google Scholar]
- Davis HP, Rosenzweig MR. Recovery as a function of the degree of amnesia due to protein synthesis inhibition. Pharmacology Biochemistry and Behavior. 1978;8:701–710. doi: 10.1016/0091-3057(78)90269-1. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychological Bulletin. 1984;96:518–559. [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Dismukes RK, Rake AV. Involvement of biogenic amines in memory formation. Psychopharmacologia. 1972;23:17–25. doi: 10.1007/BF00414410. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annual Review of Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. Journal of Neuroscience. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DR, Mahadevan LC. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms: lack of evidence for labile repressors. EMBO J. 1992;11:2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estibeiro P, Godfray J. Antisense as a neuroscience tool and therapeutic agent. Trands in Neurosciences. 2001;24:S56–S62. doi: 10.1016/s0166-2236(00)01968-8. [DOI] [PubMed] [Google Scholar]
- Flexner LB, Goodman RH. Studies on memory: inhibitors of protein synthesis also inhibit catecholamine synthesis. Proceedings of the National Acadademy of Sciences USA. 1975;72:4660–4663. doi: 10.1073/pnas.72.11.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Bennett EL, Orme AE, Rosenzweig MR, Jarvik ME. Memory: modification of anisomycin-induced amnesia by stimulants and depressants. Science. 1978;199:324–326. doi: 10.1126/science.619461. [DOI] [PubMed] [Google Scholar]
- Flood JF, Bennett EL, Orme AE, Rosenzweig MR. Relation of memory formation to controlled amounts of brain protein synthesis. Physiology & Behavior. 1975;15:97–102. doi: 10.1016/0031-9384(75)90285-1. [DOI] [PubMed] [Google Scholar]
- Flood JF, Jarvik ME, Bennett EL, Orme AE, Rosenzweig MR. Protein synthesis inhibition and memory for pole jump active avoidance and extinction. Pharmacology Biochemistry and Behavior. 1977;7:71–77. doi: 10.1016/0091-3057(77)90013-2. [DOI] [PubMed] [Google Scholar]
- Flood JF, Rosenzweig MR, Bennett EL, Orme AE. The influence of duration of protein synthesis inhibition on memory. Physiology & Behavior. 1973;10:555–562. doi: 10.1016/0031-9384(73)90221-7. [DOI] [PubMed] [Google Scholar]
- Forte A, Cipollaro M, Cascino A, Galderisi U. Small interfering RNAs and antisense oligonucleotides for treatment of neurological diseases. Current Drug Targets. 2005;6:21–29. doi: 10.2174/1389450053344920. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nature Reviews Neuroscience. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Freeman FM, Rose SP, Scholey AB. Two time windows of anisomycin-induced amnesia for passive avoidance training in the day-old chick. Neurobiology of Learning and Memory. 1995;63:291–295. doi: 10.1006/nlme.1995.1034. [DOI] [PubMed] [Google Scholar]
- Freudenthal R, Boccia MM, Acosta GB, Blake MG, Merlo E, Baratti CM, Romano A. NF-kappaB transcription factor is required for inhibitory avoidance long-term memory in mice. European Journal of Neuroscience. 2005;21:2845–2852. doi: 10.1111/j.1460-9568.2005.04126.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Osta A, Tsokas P, Pollonini G, Landau EM, Blitzer R, Alberini CM. MuSK expressed in the brain mediates cholinergic responses, synaptic plasticity, and memory formation. Journal of Neuroscience. 2006;26:7919–7932. doi: 10.1523/JNEUROSCI.1674-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. Journal of Neuroscience. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent LTP. Journal of Biological Chemistry. doi: 10.1074/jbc.M701077200. In press. [DOI] [PubMed] [Google Scholar]
- Gold PE. The many faces of amnesia. Learning and Memory. 2006;13:506–514. doi: 10.1101/lm.277406. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nicolini V, McGinty JF. Gene expression profile from the striatum of amphetamine-treated rats: a cDNA array and in situ hybridization histochemical study. Brain Research Gene Expression Patterns. 2002;1:193–198. doi: 10.1016/s1567-133x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Matthies H. Two sensitive periods for the amnesic effect of anisomycin. Pharmacology Biochemistry and Behavior. 1980;12:663–665. doi: 10.1016/0091-3057(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Grollman AP. Inhibitors of protein biosynthesis. II. Mode of action of anisomycin. Journal of Biological Chemistry. 1967;242:3226–3233. [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proceedings of the National Academy of Science USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzalin CA, Le Panse R, Cano E, Mahadevan LC. Anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction. Molecular Cell Biology. 1998;18:1844–1854. doi: 10.1128/mcb.18.4.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway BW, Ripley SH. Nucleic acid content of reticulocytes and its relation to uptake of radioactive leucine in vitro. Journal of Biological Chemistry. 1952;196:695–701. [PubMed] [Google Scholar]
- Jiménez A, Vázquez D. Anisomycin and related antibiotics. In: Hahn FE, editor. Antibiotics. pt 2. vol. 5. Springer-Verlag; New York: 1979. pp. 1–19. [Google Scholar]
- Johnston AN, Rose SP. Memory consolidation in day-old chicks requires BDNF but not NGF or NT-3; an antisense study. Brain Research Molecular Brain Research. 2001;88:26–36. doi: 10.1016/s0169-328x(01)00016-x. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nature Reviews Neuroscience. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proceedings of the National Academy of Sciences USA. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Leil TA, Ossadtchi A, Nichols TE, Leahy RM, Smith DJ. Genes regulated by learning in the hippocampus. Journal of Neuroscience Research. 2003;71:763–768. doi: 10.1002/jnr.10541. [DOI] [PubMed] [Google Scholar]
- Lewis DJ. Psychobiology of active and inactive memory. Psychological Bulletin. 1979;5:1054–1083. [PubMed] [Google Scholar]
- Li MD, Kane JK, Wang J, Ma JZ. Time-dependent changes in transcriptional profiles within five rat brain regions in response to nicotine treatment. Brain Research Molecular Brain Research. 2004;132:168–180. doi: 10.1016/j.molbrainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Lieberman I, Abrams R, Hunt N, Ove P. Levels of enzyme activity and deoxyribonucleic acid synthesis in mammalian cells cultured from the animal. Journal of Biological Chemistry. 1963;238:3955–3962. [PubMed] [Google Scholar]
- Mahadevan LC, Edwards DR. Signalling and superinduction. Nature. 1991;349:747–748. doi: 10.1038/349747c0. [DOI] [PubMed] [Google Scholar]
- Martin KC, Zukin RS. RNA trafficking and local protein synthesis in dendrites: an overview. Journal of Neuroscience. 2006;26:7131–7134. doi: 10.1523/JNEUROSCI.1801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Green DR, Cotter TG. Dicing with death: dissecting the components of the apoptosis machinery. Trends in Biochemical Sciences. 1994;19:26–30. doi: 10.1016/0968-0004(94)90170-8. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McNair K, Broad J, Riedel G, Davies CH, Cobb SR. Global changes in the hippocampal proteome following exposure to an enriched environment. Neuroscience. 2007;145:413–422. doi: 10.1016/j.neuroscience.2006.12.033. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Pollonini G, Alberini CM. Temporal requirement of C/EBPbeta in the amygdala following reactivation but not acquisition of inhibitory avoidance. Learning and Memory. 2007 doi: 10.1101/lm.598307. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. Journal of Neuroscience. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Amnesia or retrieval deficit? Implications of a molecular approach to the question of reconsolidation. Learning and Memory. 2006;13:498–505. doi: 10.1101/lm.304606. [DOI] [PubMed] [Google Scholar]
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joels M, De Kloet ER, Datson NA. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. Journal of Neuroendocrinology. 2006;18:239–252. doi: 10.1111/j.1365-2826.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- Mueller GC, Kajiwara K, Stubblefield E, Rueckert RR. Molecular events in the reproduction of animal cells. I. The effect of puromycin on the duplication of DNA. Cancer Research. 1962;22:1084–1090. [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nature Reviews Neuroscience. 2000;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Obrig TG, Culp WJ, McKeehan WL, Hardesty B. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. Journal of Biological Chemistry. 1971;246:174–181. [PubMed] [Google Scholar]
- O'Sullivan NC, McGettigan PA, Sheridan GK, Pickering M, Conboy L, O'Connor JJ, Moynagh PN, Higgins DG, Regan CM, Murphy KJ. Temporal change in gene expression in the rat dentate gyrus following passive avoidance learning. Journal of Neurochemistry. 2007;101:1085–1098. doi: 10.1111/j.1471-4159.2006.04418.x. [DOI] [PubMed] [Google Scholar]
- Park JS, Onodera T, Nishimura S, Thompson RF, Itohara S. Molecular evidence for two-stage learning and partial laterality in eyeblink conditioning of mice. Proceeding of the National Academy of Science U S A. 2006;103:5549–5554. doi: 10.1073/pnas.0601150103. Epub 2006 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block "reconsolidation" but impairs extinction: the role of re-exposure duration. Learning and Memory. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartermain D, Botwinick CY. Role of the biogenic amines in the reversal of cycloheximide-induced amnesia. Journal of Comparative Physiology and Psychology. 1975;88:386–401. doi: 10.1037/h0076208. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Vianna MR, Roesler R, de-Paris F, Izquierdo I, Rose SP. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learning and Memory. 1999;6:600–607. doi: 10.1101/lm.6.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Vianna MR, Roesler R, Martins MR, de-Paris F, Medina JH, Izquierdo I. Pretraining but not preexposure to the task apparatus prevents the memory impairment induced by blockade of protein synthesis, PKA or MAP kinase in rats. Neurochemical Research. 2005;30:61–67. doi: 10.1007/s11064-004-9686-3. [DOI] [PubMed] [Google Scholar]
- Randt CT, Quartermain D, Goldstein M, Anagnoste B. Norepinephrine biosynthesis inhibition: effects on memory in mice. Science. 1971;172:498–499. doi: 10.1126/science.172.3982.498. [DOI] [PubMed] [Google Scholar]
- Rao SS, Grollman AP. Cycloheximide resistance in yeast: a property of the 60S ribosomal subunit. Biochemical and Biophysical Research Communications. 1967;29:696–704. doi: 10.1016/0006-291x(67)90273-2. [DOI] [PubMed] [Google Scholar]
- Rehen SK, Varella MH, Freitas FG, Moraes MO, Linden R. Contrasting effects of protein synthesis inhibition and of cyclic AMP on apoptosis in the developing retina. Development. 1996;122:1439–1448. doi: 10.1242/dev.122.5.1439. [DOI] [PubMed] [Google Scholar]
- Riccio DC, Millin PM, Bogart AR. Reconsolidation: a brief history, a retrieval view, and some recent issues. Learning and Memory. 2006;13:536–544. doi: 10.1101/lm.290706. [DOI] [PubMed] [Google Scholar]
- Riccio DC, Moody EW, Millin PM. Reconsolidation reconsidered. Integrative Physiological and Behavioral Science. 2002;37:245–253. doi: 10.1007/BF02734247. [DOI] [PubMed] [Google Scholar]
- Richter JD. CPEB: a life in translation. Trends in Biochemical Sciences. 2007;32:279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ronnback L, Hansson E. Stimulation of brain-stem protein synthesis by morphine. Biochemical Pharmacology. 1986;35:3685–3692. doi: 10.1016/0006-2952(86)90652-0. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends in Neuroscience. 2005;28:12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, Moineau J, Bolding K. Anisomycin and the reconsolidation hypothesis. Learning and Memory. 2006;13:1–3. doi: 10.1101/lm.157806. [DOI] [PubMed] [Google Scholar]
- Salinska E, Bourne RC, Rose SP. Reminder effects: the molecular cascade following a reminder in young chicks does not recapitulate that following training on a passive avoidance task. European Journal of Neuroscience. 2004;19:3042–3047. doi: 10.1111/j.0953-816X.2004.03407.x. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learning and Memory. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Sidhu JS, Omiecinski CJ. Protein synthesis inhibitors exhibit a nonspecific effect on phenobarbital-inducible cytochome P450 gene expression in primary rat hepatocytes. Journal of Biological Chemistry. 1998;273:4769–4775. doi: 10.1074/jbc.273.8.4769. [DOI] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Sokolov BP, Polesskaya OO, Uhl GR. Mouse brain gene expression changes after acute and chronic amphetamine. Journal of Neurochemistry. 2003;84:244–252. doi: 10.1046/j.1471-4159.2003.01523.x. [DOI] [PubMed] [Google Scholar]
- Squire LR. Lost forever or temporarily misplaced? The long debate about the nature of memory impairment. Learning and Memory. 2007;13:522–529. doi: 10.1101/lm.310306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Current Opinion Neurobiology. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Subramaniam M, Schmidt LJ, Crutchfield CE, 3rd, Getz MJ. Negative regulation of serum-responsive enhancer elements. Nature. 1989;340:64–66. doi: 10.1038/340064a0. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. Journal of Neuroscience. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Monti B, Dolan B, Pollonini G, Alberini CM. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein [beta] and [delta] Co-localizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. Journal of Neuroscience. 2001a;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPbeta. Nature Neuroscience. 2001b;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Tessitore L, Tomasi C, Greco M. Fasting-induced apoptosis in rat liver is blocked by cycloheximide. European Journal of Cell Biology. 1999;78:573–579. doi: 10.1016/S0171-9335(99)80023-5. [DOI] [PubMed] [Google Scholar]
- Tirosh S, Elkobi A, Rosenblum K, Meiri N. A role for eukaryotic translation initiation factor 2B (eIF2B) in taste memory consolidation and in thermal control establishment during the critical period for sensory development. Developmental Neurobiology. 2007;67:728–739. doi: 10.1002/dneu.20378. [DOI] [PubMed] [Google Scholar]
- Torocsik B, Szeberenyi J. Anisomycin affects both pro- and antiapoptotic mechanisms in PC12 cells. Biochem Biophys Research Communication 2000. 2000;278:550–556. doi: 10.1006/bbrc.2000.3836. [DOI] [PubMed] [Google Scholar]
- Tronel S, Milekic MH, Alberini CM. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biology. 2005;3:e293. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulzsch B, Wood M. Applications of nucleic acid technology in the CNS. Journal of Neurochemistry. 2004;88:257–265. doi: 10.1111/j.1471-4159.2004.02153.x. [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]


