Abstract
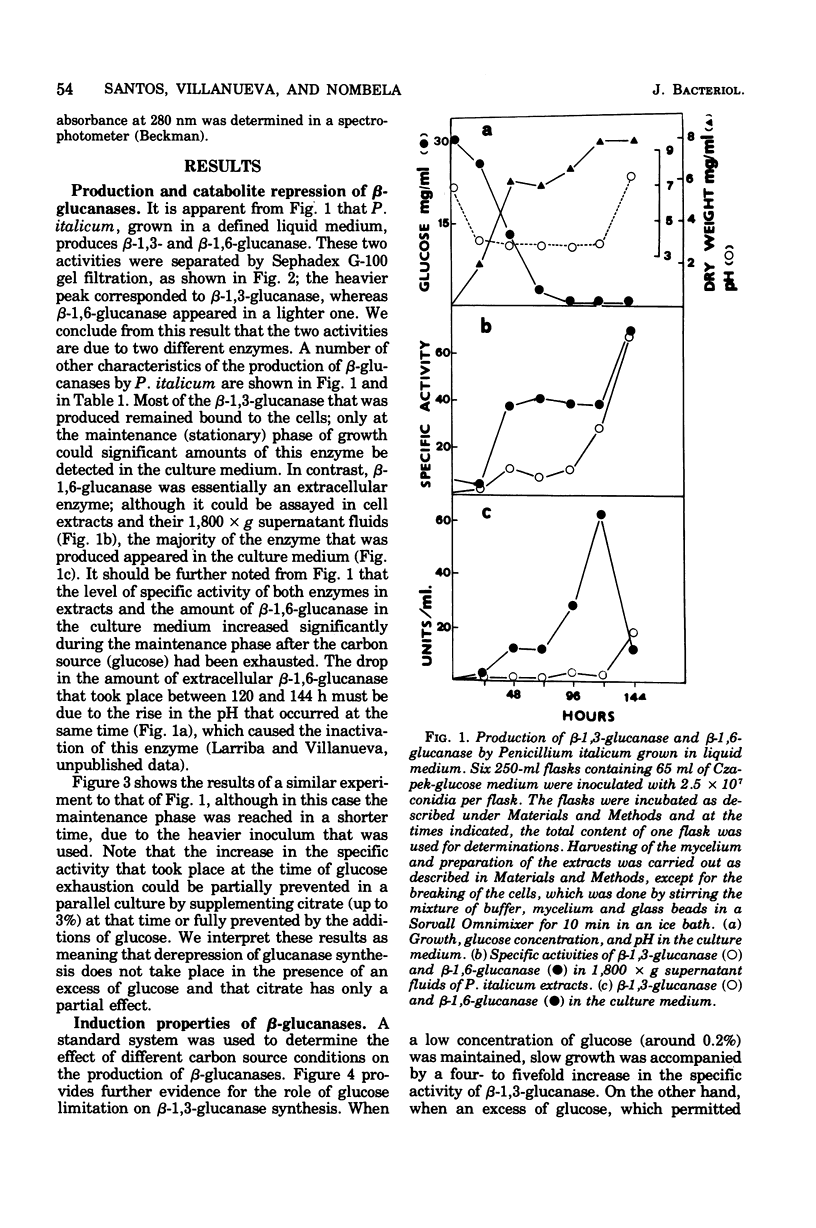
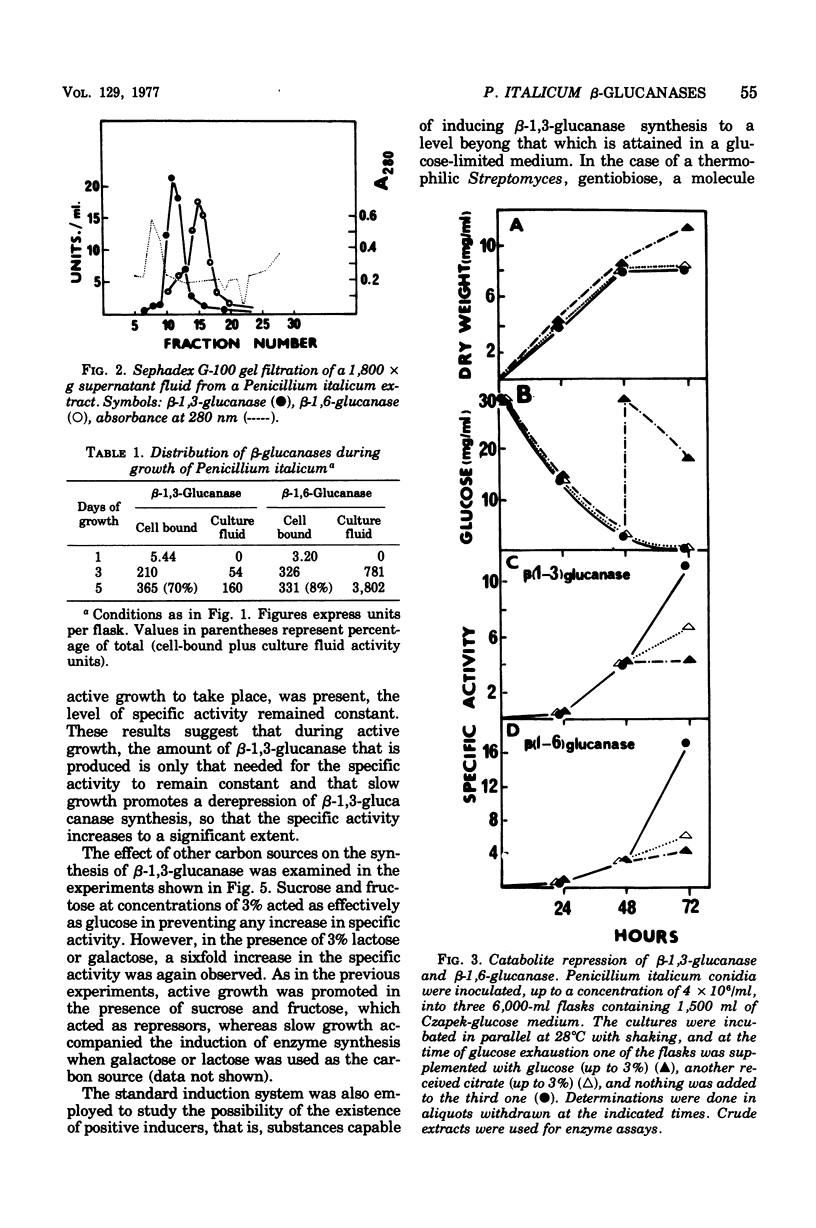
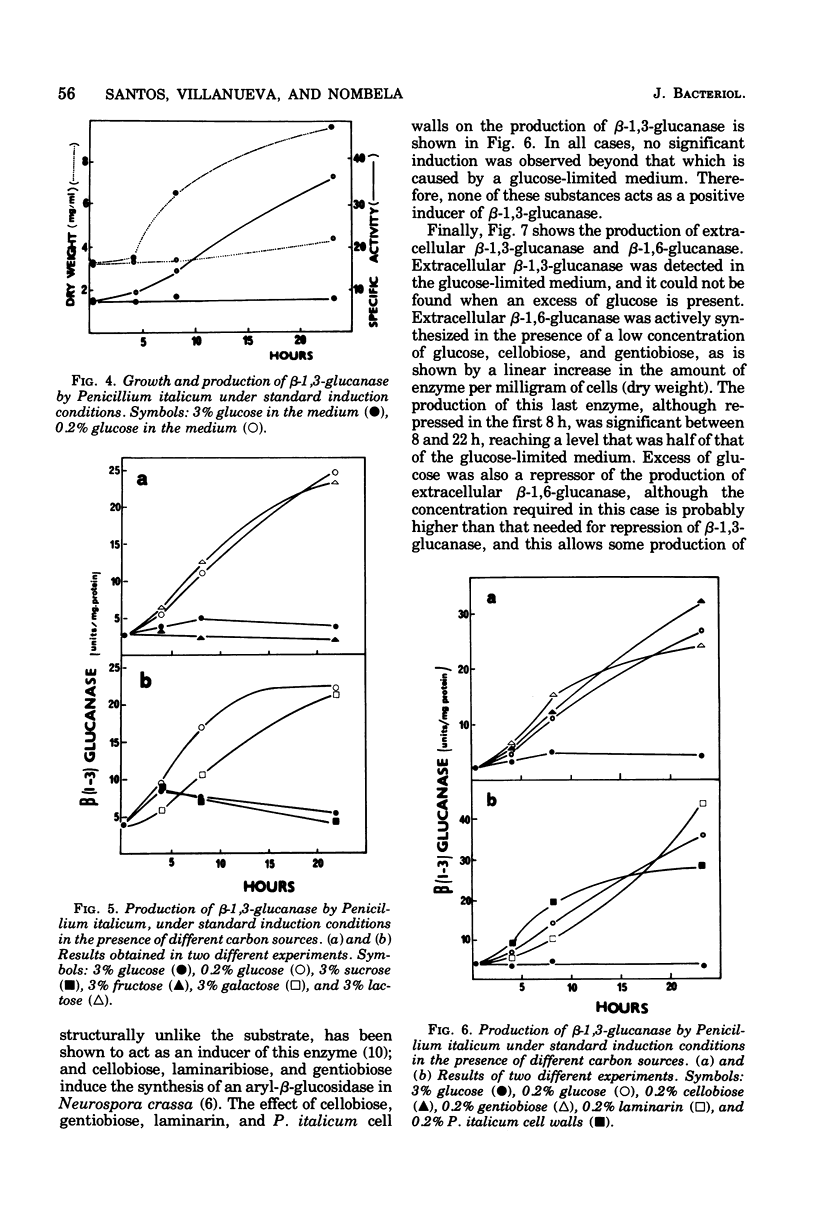
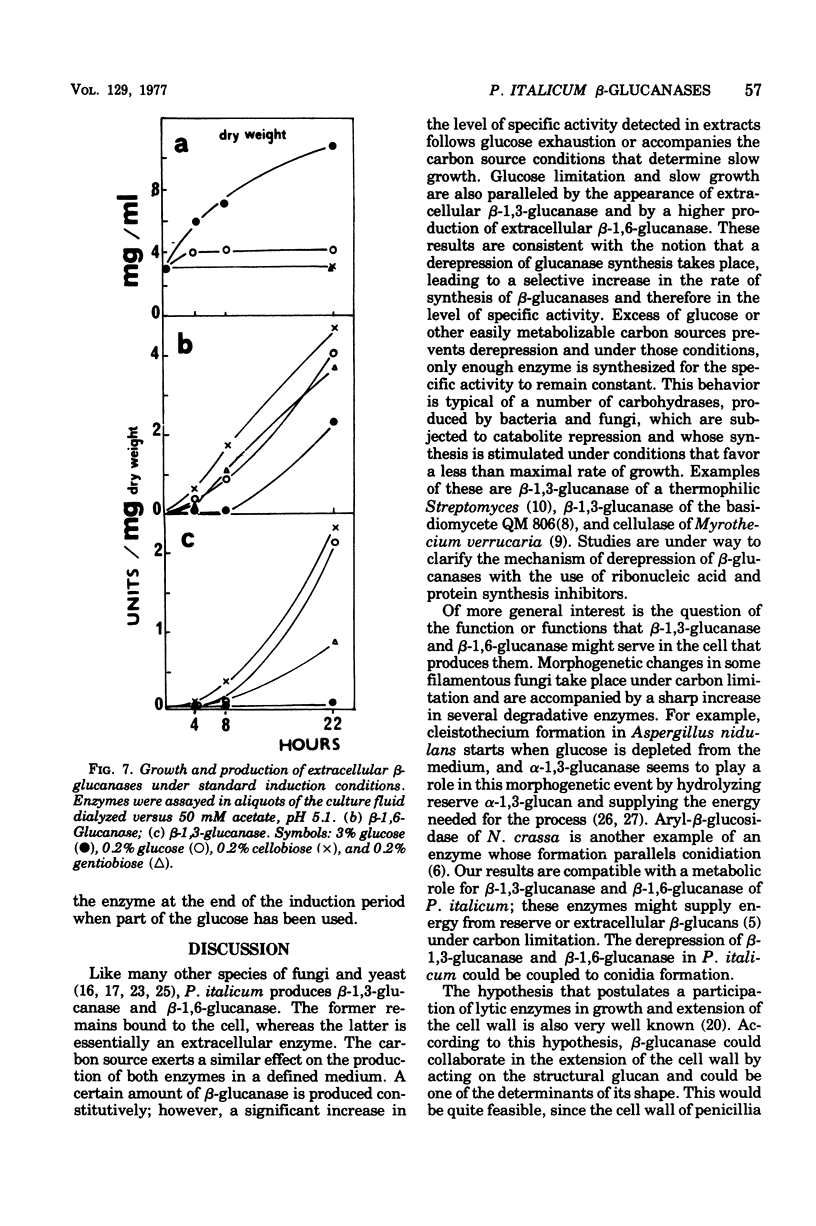
The filamentous fungus Penicillium italicum, grown in a defined liquid medium, produced beta-1,3-glucanase, which remained essentially bound to the cells, and beta-1,6-glucanase, an essentially extracellular enzyme. When glucose was depleted from the medium, when a limited concentration of glucose (0.2%) was maintained, or when the carbon source was galactose (3%) or lactose (3%), a significant increase in the specific activity of beta-1,3-glucanase, in cell extracts, took place. This was paralleled by a very slow rate of growth, and under glucose limitation, the appearance of beta-1,3-glucanase in the medium was also observed. On the other hand, when an excess of glucose, fructose, or sucrose was present, the specific activity remained constant and active growth was promoted. Laminarin, cellobiose, gentiobiose, and isolated Penicillium italicum walls were not capable of significantly inducing beta-1,3-glucanase synthesis to a level beyond that attained by glucose limitation. A similar behavior was observed for beta-1,6-glucanase. beta-1,3-Glucanase and beta-1,6-glucanase are therefore constitutive enzymes subjected to catabolite repression. The results are discussed in the context of the possible functions that have been suggested for glucanases and related enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A. T., Phaff H. J. Exo-beta-glucanases in yeast. Biochem J. 1968 Sep;109(3):347–360. doi: 10.1042/bj1090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biely P., Farkas V., Bauer S. Secretion of -glucanase by Saccharomyces cerevisiae protoplasts. FEBS Lett. 1972 Jun 15;23(2):153–156. doi: 10.1016/0014-5793(72)80328-4. [DOI] [PubMed] [Google Scholar]
- CHESTERS C. G., BULL A. T. The enzymic degradation of laminarin. 1. The distribution of laminarinase among micro-organisms. Biochem J. 1963 Jan;86:28–31. doi: 10.1042/bj0860028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson A. G., Mantle P. G., Szczyrbak C. A. Autolysis of extracellular glucans produced in vitro by a strain of Claviceps fusiformis. J Gen Microbiol. 1970 Mar;60(3):403–415. doi: 10.1099/00221287-60-3-403. [DOI] [PubMed] [Google Scholar]
- Eberhart B. M., Beck R. S. Induction of beta-glucosidases in Neurospora crassa. J Bacteriol. 1973 Oct;116(1):295–303. doi: 10.1128/jb.116.1.295-303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas V., Biely P., Bauer S. Extracellular beta-glucanases of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Sep 15;321(1):246–255. doi: 10.1016/0005-2744(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Friebe B., Holldorf A. W. Control of Extracellular beta-1,3-glucanase activity in a basidiomycete species. J Bacteriol. 1975 Jun;122(3):818–825. doi: 10.1128/jb.122.3.818-825.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme M. A., Stranks D. W. Regulation of cellulase production by Myrothecium verrucaria grown on non-cellulosic substrates. J Gen Microbiol. 1971 Dec;69(2):145–155. doi: 10.1099/00221287-69-2-145. [DOI] [PubMed] [Google Scholar]
- Lilley G., Bull A. T. The production of beta-1,3 glucanase by a thermophilic species of streptomyces. J Gen Microbiol. 1974 Jul;83(0):123–133. doi: 10.1099/00221287-83-1-123. [DOI] [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Relationship of the major constituents of the Neurospora crassa cell wall to wild-type and colonial morphology. J Bacteriol. 1965 Oct;90(4):1073–1081. doi: 10.1128/jb.90.4.1073-1081.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monreal J., De Uruburu F., Villanueva J. R. Lytic action of beta-(1-3)-glucanase on yeast cells. J Bacteriol. 1967 Jul;94(1):241–244. doi: 10.1128/jb.94.1.241-244.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REESE E. T., MANDELS M. Beta-D-1, 3 Glucanases in fungi. Can J Microbiol. 1959 Apr;5(2):173–185. doi: 10.1139/m59-022. [DOI] [PubMed] [Google Scholar]
- REESE E. T., PARRISH F. W., MANDELS M. Beta-d-1, 6-Glucanases in fungi. Can J Microbiol. 1962 Jun;8:327–334. doi: 10.1139/m62-045. [DOI] [PubMed] [Google Scholar]
- Rombouts F. M., Phaff H. J. Lysis of yeast cell walls. Lytic beta-(1 leads to 3)-glucanases from Bacillus circulans WL-12. Eur J Biochem. 1976 Mar 16;63(1):121–130. doi: 10.1111/j.1432-1033.1976.tb10214.x. [DOI] [PubMed] [Google Scholar]
- Rombouts F. M., Phaff H. J. Lysis of yeast cell walls. Lytic beta-(1 leads to 6)-glucanase from Bacillus circulans WL-12. Eur J Biochem. 1976 Mar 16;63(1):109–120. doi: 10.1111/j.1432-1033.1976.tb10213.x. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Troy F. A., Koffler H. The chemistry and molecular architecture of the cell walls of Penicillium chrysogenum. J Biol Chem. 1969 Oct 25;244(20):5563–5576. [PubMed] [Google Scholar]
- Villa T. G., Notario V., Villanueva J. R. Beta-glucanases of the yeast Pichia polymorpha. Arch Microbiol. 1975 Jun 22;104(2):201–206. doi: 10.1007/BF00447325. [DOI] [PubMed] [Google Scholar]
- Zonneveld B. J. Inhibitory effect of 2-Deoxyglucose on cell wall alpha-1,3-glucan synthesis and cleistothecium development in Aspergillus nidulans. Dev Biol. 1973 Sep;34(1):1–8. doi: 10.1016/0012-1606(73)90334-5. [DOI] [PubMed] [Google Scholar]
- Zonneveld B. J. Morphogenesis in Aspergillus nidulans. The significance of a alpha-1, 3-glucan of the cell wall and alpha-1, 3-glucanase for cleistothecium development. Biochim Biophys Acta. 1972 Jun 26;273(1):174–187. doi: 10.1016/0304-4165(72)90205-x. [DOI] [PubMed] [Google Scholar]


