Abstract
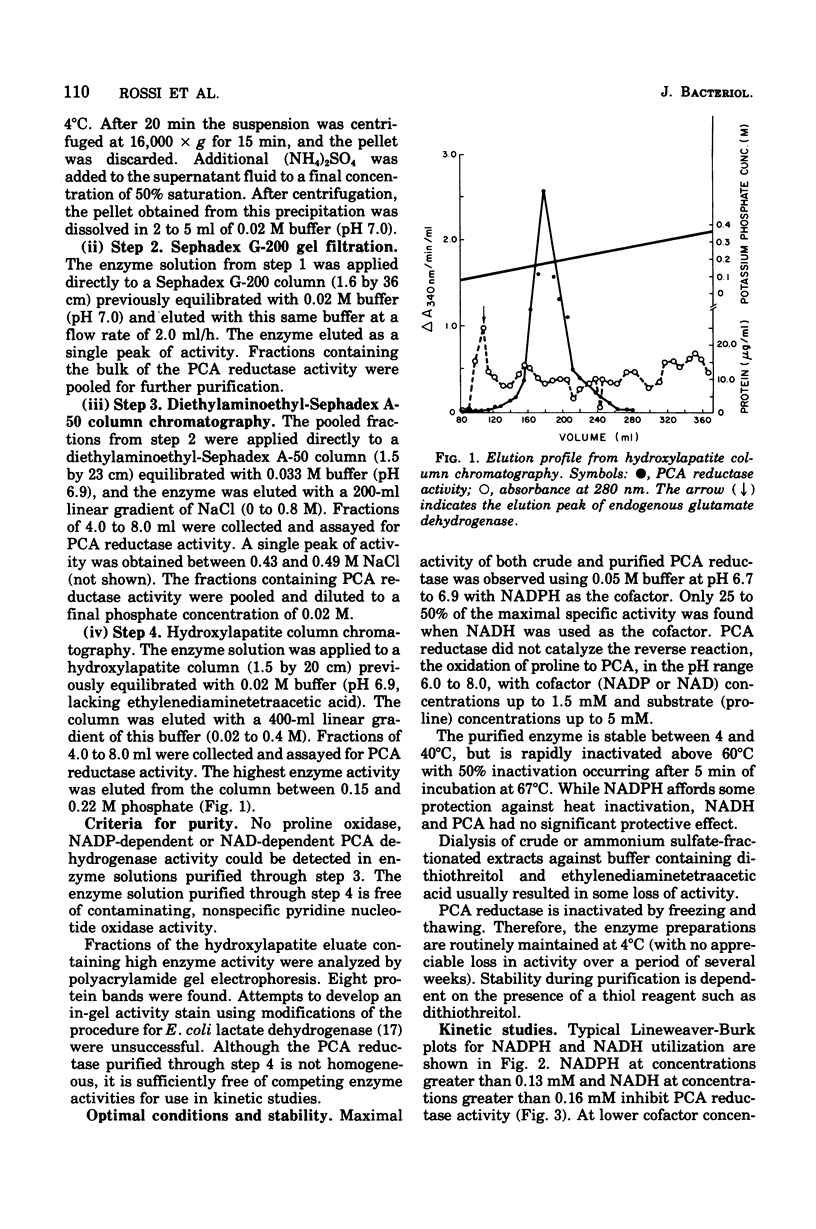
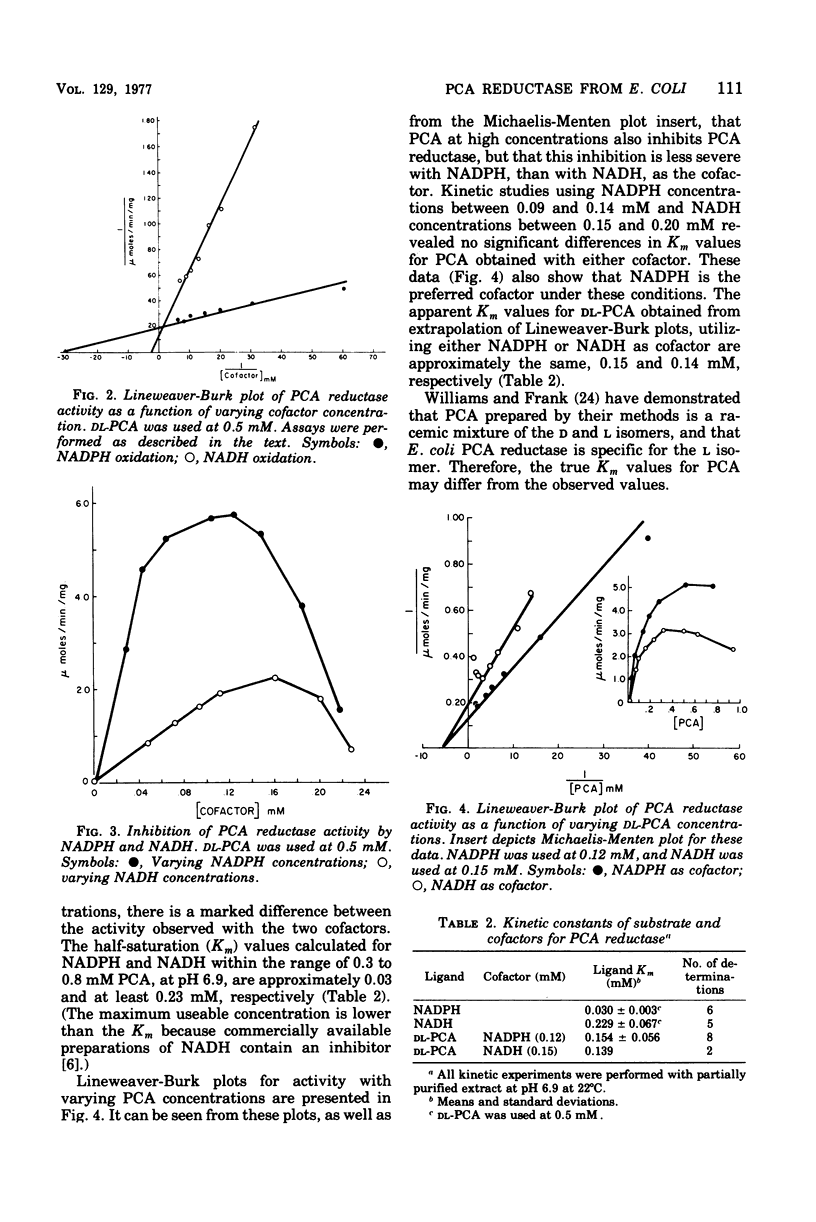
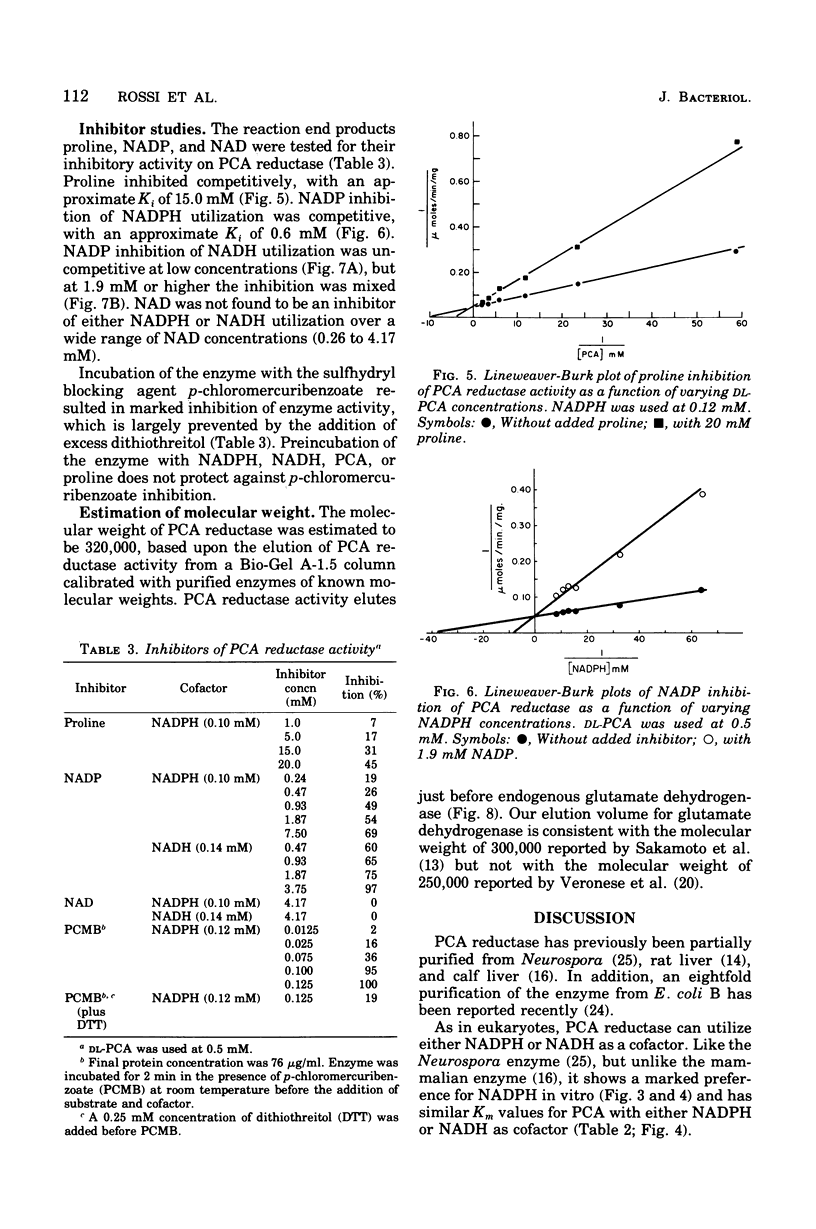
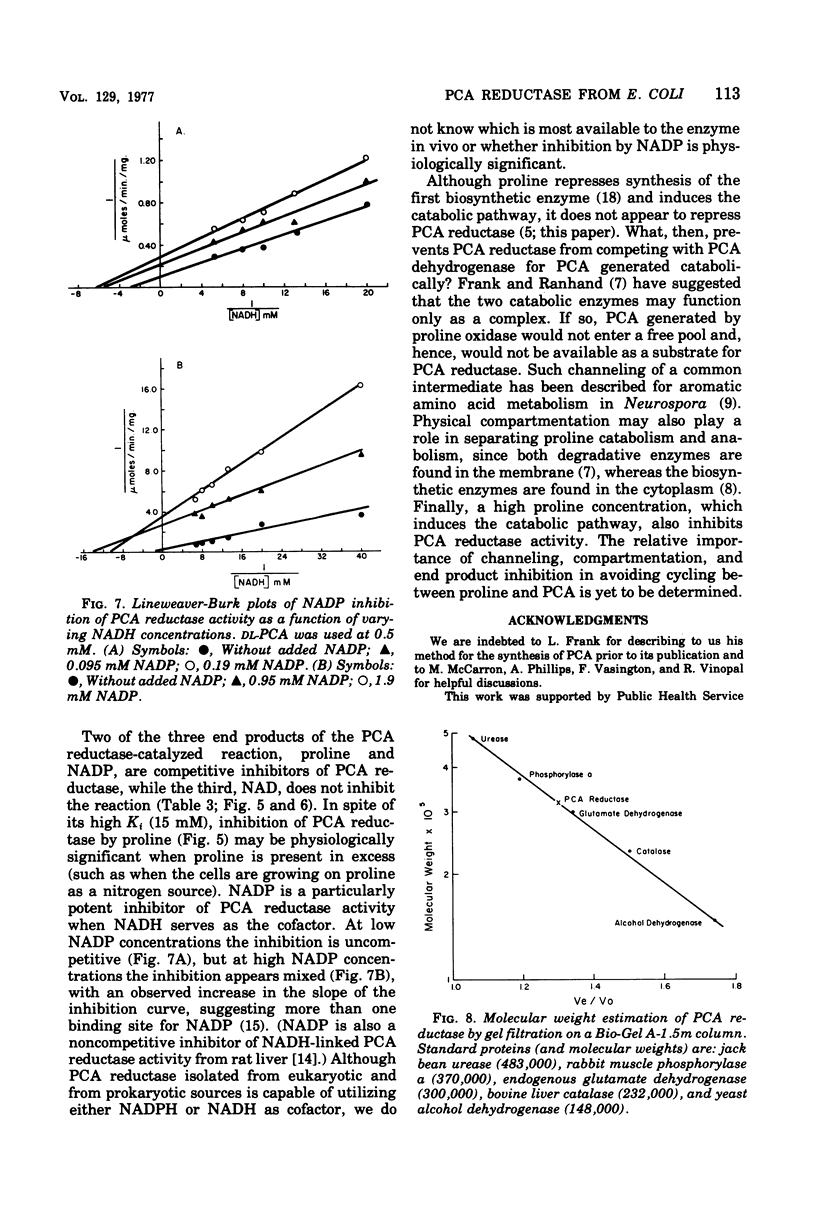
delta1-Pyrroline-5-carboxylate (PCA) reductase [L-proline:NAD(P)+5-oxidoreductase, EC 1.5.1.2] has been purified over 200-fold from Escherichia coli K-12. It has a molecular weight of approximately 320,000. PCA reductase mediates the pyridine nucleotide-linked reduction of PCA to proline but not the reverse reaction (even at high substrate concentrations). The partially purified preparation is free of competing pyridine nucleotide oxidase, PCA dehydrogenase, and proline oxidase activities. The Michaelis constant (Km) values for the substrate, PCA, with reduced nicotinamide adenine dinucleotide phosphate (NADPH) or NADH as cofactor are 0.15 and 0.14 mM, respectively. The Km values determined for NADPH and NADH are 0.03 and 0.23 mM, respectively. Although either NADPH or NADH can function as cofactor, the activity observed with NADPH is severalfold greater. PCA reductase is not repressed by growth in the presence of proline, but it is inhibited by the reaction end products, proline and NADP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baich A., Pierson D. J. Control of proline synthesis in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):397–404. doi: 10.1016/0304-4165(65)90345-4. [DOI] [PubMed] [Google Scholar]
- Baich A. Proline synthesis in Escherichia coli. A proline-inhibitable glutamic acid kinase. Biochim Biophys Acta. 1969 Dec 30;192(3):462–467. doi: 10.1016/0304-4165(69)90395-x. [DOI] [PubMed] [Google Scholar]
- Baich A. The biosynthesis of proline in Escherichia coli: phosphate-dependent glutamate -semialdehyde dehydrogenase (NADP), the second enzyme in the pathway. Biochim Biophys Acta. 1971 Jul 20;244(1):129–134. doi: 10.1016/0304-4165(71)90129-2. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Rossi J. J. Proline excretion and indirect suppression in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1974 Jun;118(3):928–934. doi: 10.1128/jb.118.3.928-934.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine H. Sur la régulation de la production de proline chez E. Coli K 12. Ann Inst Pasteur (Paris) 1971 Feb;120(2):126–143. [PubMed] [Google Scholar]
- FAWCETT C. P., CIOTTI M. M., KAPLAN N. O. Inhibition of dehydrogenase reactions by a substance formed from reduced diphosphopyridine nucleotide. Biochim Biophys Acta. 1961 Nov 25;54:210–212. doi: 10.1016/0006-3002(61)90962-3. [DOI] [PubMed] [Google Scholar]
- FRANK L., RANHAND B. PROLINE METABOLISM IN ESCHERICHIA COLI. 3. THE PROLINE CATABOLIC PATHWAY. Arch Biochem Biophys. 1964 Aug;107:325–331. doi: 10.1016/0003-9861(64)90338-8. [DOI] [PubMed] [Google Scholar]
- Gamper H., Moses V. Enzyme organization in the proline biosynthetic pathway of Escherichia coli. Biochim Biophys Acta. 1974 Jun 20;354(1):75–87. doi: 10.1016/0304-4165(74)90055-5. [DOI] [PubMed] [Google Scholar]
- Giles N. H., Partridge C. W., Ahmed S. I., Case M. E. The occurrence of two dehydroquinases in Neurospora crassa, one constitutive and one inducible. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1930–1937. doi: 10.1073/pnas.58.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON A. B., STRECKER H. J. The interconversion of glutamic acid and proline. IV. The oxidation of proline by rat liver mitochondria. J Biol Chem. 1962 Jun;237:1876–1882. [PubMed] [Google Scholar]
- PEISACH J., STRECKER H. J. The interconversion of glutamic acid and proline. V. The reduction of delta 1-pyrroline-5-carboxylic acid to proline. J Biol Chem. 1962 Jul;237:2255–2260. [PubMed] [Google Scholar]
- Rossi J. J., Berg C. M. Differential recovery of auxotrophs after penicillin enrichment in Escherichia coli. J Bacteriol. 1971 May;106(2):297–300. doi: 10.1128/jb.106.2.297-300.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH M. E., GREENBERG D. M. Preparation and properties of partially purified glutamic semialdehyde reductase. J Biol Chem. 1957 May;226(1):317–327. [PubMed] [Google Scholar]
- Sakamoto N., Kotre A. M., Savageau M. A. Glutamate dehydrogenase from Escherichia coli: purification and properties. J Bacteriol. 1975 Nov;124(2):775–783. doi: 10.1128/jb.124.2.775-783.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- Vender J., Jayaraman K., Rickenberg H. V. Metabolism of glutamic acid in a mutant of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1304–1307. doi: 10.1128/jb.90.5.1304-1307.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. M., Boccu E., Conventi L. Glutamate dehydrogenase from Escherichia coli: induction, purification and properties of the enzyme. Biochim Biophys Acta. 1975 Feb 19;377(2):217–228. doi: 10.1016/0005-2744(75)90304-6. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Williams I., Frank L. Improved chemical synthesis and enzymatic assay of delta-1-pyrroline-5-carboxylic acid. Anal Biochem. 1975 Mar;64(1):85–97. doi: 10.1016/0003-2697(75)90408-x. [DOI] [PubMed] [Google Scholar]
- YURA T., VOGEL H. J. Pyrroline-5-carboxylate reductase of Neurospora crassa; partial purification and some properties. J Biol Chem. 1959 Feb;234(2):335–338. [PubMed] [Google Scholar]


