Abstract
Inferences about how the complex sensory and motor systems of the human brain evolved are based on the results of comparative studies of brain organization across a range of mammalian species, and evidence from the endocasts of fossil skulls of key extinct species. The endocasts of the skulls of early mammals indicate that they had small brains with little neocortex. Evidence from comparative studies of cortical organization from small-brained mammals of the six major branches of mammalian evolution supports the conclusion that the small neocortex of early mammals was divided into roughly 20–25 cortical areas, including primary and secondary sensory fields. In early primates, vision was the dominant sense, and cortical areas associated with vision in temporal and occipital cortex underwent a significant expansion. Comparative studies indicate that early primates had 10 or more visual areas, and somatosensory areas with expanded representations of the forepaw. Posterior parietal cortex was also expanded, with a caudal half dominated by visual inputs, and a rostral half dominated by somatosensory inputs with outputs to an array of seven or more motor and visuomotor areas of the frontal lobe. Somatosensory areas and posterior parietal cortex became further differentiated in early anthropoid primates. As larger brains evolved in early apes and in our hominin ancestors, the number of cortical areas increased to reach an estimated 200 or so in present day humans, and hemispheric specializations emerged. The large human brain grew primarily by increasing neuron number rather than increasing average neuron size.
Introduction
One cannot help but be impressed with the sensorimotor skills of our best athletes and musicians. Their remarkable abilities are usually the result of years of training and practice, often starting in childhood. Yet, most of us are also able to acquire unusual skills with training and experience. While the exceptional athlete or musician may have advantages of several sorts, including those related to body morphology and physiology, and possibly favorable variations in brain organization and function, the human brain is capable of acquiring and mediating sensorimotor abilities that are unmatched in other species. It is somewhat uncertain why this is so, but an obvious factor is our large brain, with densely packed with neurons [10], which is largely devoted to processing and storing sensory and other information and using it to guide, plan and mediate behaviors. While this large brain emerged gradually from the time of our early mammal ancestors, who had small brains and little neocortex [22], the most dramatic increase in size occurred over the last two million years. However, the increase in brain size only partially accounts for human abilities, as the organization of the human brain changed greatly from the time of early mammals. The changes in brain organization that did occur are not fully understood, but some of the probable changes can be inferred from what is known about brain organization in extant mammals.
Currently, our best indications of how the human brain is organized come from studies of the brains of other primates, most often Old World macaque monkeys. Studies of these and other monkeys indicate that their sensory and motor systems are exceedingly complex, particularly at the cortical level of processing. In these monkeys, the cortical sheet is divided into a large number of functionally distinct processing organs, the cortical areas. In turn, cortical areas are often, and perhaps typically, further divided into two or more sets of modules or columns of functionally related neurons, so that single areas can mediate several distinct, but related functions. The cortical areas are interconnected most densely with adjacent areas that have related functions, and interconnected arrays of such areas form cortical systems. Systems of related areas also interconnect and relate to other systems. The great computational power of these brains comes from the reiteration of basic processing steps from one area to another to produce rather complex computations. In this regard, the processing at the cortical level in macaque monkeys involves 30–40 areas that are predominately visual, 15–20 auditory areas, 15–20 somatosensory areas, and 10 or more motor areas [8,18,20,28,32]. The exact numbers are uncertain, as it can be difficult to experimentally identify cortical areas, especially higher order cortical areas. Thus, there is widespread agreement on the identity of relatively few areas. Current research has been leading to new proposals where boundaries of previously proposed areas have been adjusted, areas subdivided, and areas or parts of areas incorporated into new domains. Nevertheless, there is agreement that the numbers of areas given above are approximately correct, and that each area is interconnected with a number of other areas and subcortical structures to form very complex networks or hierarchies of areas within and across systems.
The 1.2–1.8 kg human brain with about 100 billion neurons is many times larger than the 0.09 kg macaque monkey brain with 6.4 billion neurons [10]. This suggests that the complexity of the human brain greatly exceeds that of the macaque monkey brain. While information about how the human brain is organized is rapidly being acquired, especially by non-invasive functional imaging techniques, we presently know less about the areal and modular organization of human brains than macaque brains, and much less about connection patterns. Yet, some estimate that the human brain has well over 200 cortical areas. The question addressed here is how did such complexity evolve? While this challenging question cannot be fully addressed here, we can gain some insights by examining the comparative data on somatosensory and motor systems, since these systems have been extensively studied [17,18]. Therefore, this review focuses on these systems, especially at the cortical level.
The nature of the problem: how do we reconstruct the past?
Normally, we can learn much about the evolution of mammals from the fossil record, which consists mainly of fossilized bone. Unfortunately, the soft tissue of the brain does not fossilize like the bone of the skull does. Fortunately, the interior of the skull often reflects the size and shape of the brain fairly well, and even some of the fissures of the brain can be apparent as protrusions on the skull interior. Brain size, especially relative to body size, provides information about behavior capacity [13]. Because the location of fissures are related to the areal organization of cortex, preserved fissure patterns may indicate the locations of some of the areas of the brain, or even somatotopic sectors of areas of somatosensory cortex [43]. Finally, the relative proportions of the frontal, temporal, parietal and occipital regions of cortex tell us something about what cortical systems were emphasized. However, the important details of how brains were organized are not preserved in the fossil record. Thus, most of what we know about brain evolution in mammals has been inferred from the results of comparative studies of brain organization [40].
Considerable theoretical progress has been made over the last 20–30 years in how to best infer ancestral states from studies of extant (currently existing) mammals [1,44,45]. Simply put, if a feature or trait is present in all members of a group of phylogenetically related mammals, then it is parsimonious to infer that the common ancestor of the group also had that trait, and that it was retained in all the preserved lines of evolution from that ancestor. This is called a cladistic analysis or reconstruction, as a group of animals stemming from a common ancestor is called a clade. The process gets a bit more complicated when only some of the extant members of the group have the trait (or character), but the rule of parsimony, the most logical explanation of the distribution of the character, holds. The method works best when the presence or absence of a character has been determined for all or most members of a clade. However, this type of analysis becomes difficult if we want to determine what traits existed in the common ancestor of all primates, because there are over 220 species of primates, many of which are unavailable for studies of brain organization. In addition, it can be difficult, costly, and labor intensive to identify many brain characters [16]. Even identifying such a prominent feature as the presence of primary somatosensory cortex can be challenging, since a reliable identification would require congruent histological, connectional, and physiological evidence from a number of primates. While histological (architectonic) evidence is easiest to obtain, it can be inconclusive. For example, S1 or parts of S1 (area 3b) have been repeatedly misidentified as other areas in various architectonic studies. The result of such difficulties is that theories of brain evolution are often constructed on partial and patchy evidence. However, as in other fields, as more evidence is gathered, theories can be altered and corrected. In view of the difficulty in studying many members of the primate clade, we need to carefully select the ones we study in detail. Results from all members of a clade are not equally informative. If the human brain has over 200 areas, for example, it would be difficult to infer from studies of the human brain alone, which of these areas, if any, have been retained from the ancestral, early primates.
What were the brains of early mammals like?
We know from the fossil record that there was variability in the phenotypes of early mammals. Yet, most were small, on the order of mouse to cat in size, and all had small brains for their body size. Most notably, these mammals had small forebrains with little neocortex. The forebrain was dominated by an olfactory bulb, and olfactory (piriform) cortex that appeared large relative to the small amount of neocortex. The skull endocasts of early mammals do not indicate how neocortex, the most impressive feature of the human brain, was organized. However, all extant mammals have a neocortex that is thick, and has a laminar organization that traditionally includes six layers. In addition, activating inputs from the thalamus or other cortical areas converge on a few neurons in layer 4 (this may not be the case for cetaceans), and dense vertical connections spread information to other layers, where it is transformed and sent to other cortical areas and subcortical structures. We do not know how neocortex emerged in the forebrains of the ancestors of mammals, as extant reptiles and birds arose from an early branch of the amniote radiation, and the therapsids in the other branch that led to mammals did not survive. The dorsal cortex in reptiles, the homolog of neocortex in mammals, is proportionally nearly as large in surface area as the neocortex of early mammals, but thinner, consisting largely of a single layer of pyramidal neurons with activating inputs terminating widely and superficially on apical dendrites [41]. Thus, the basic organization of the neuronal circuits is much different in neocortex of mammals than in dorsal cortex of reptiles, and these structures therefore have much different functions. However, we do not know how the structural and functional differences between dorsal cortex and neocortex emerged.
The other feature of neocortex of early mammals that we can infer from studies of extant mammals is that early mammals had few cortical areas, and these included primary and secondary sensory fields. This inference is made by considering the cortical organization of small-brained mammals in the six major branches of the mammalian clade [31]. In the monotreme (prototherian) superorder, cortical organization has been explored in the duck-billed platypus and echidna [25]. Both have a small primary visual area (V1), a primary somatosensory area, with a rostral field, R, and a lateral somatosensory field termed PV. A primary auditory field (A1), and possibly other auditory fields, is present. There is some evidence for a primary motor field, but the existence of this field is questionable. In the marsupial (metatherian) superorder, we find clear evidence for these same few sensory fields [24], but the bulk of the evidence argues against the presence of a primary motor field [2]. As in other mammals, somatosensory cortex of marsupial opossums has motor functions. In addition, there is compelling evidence for the presence of a second visual area, V2, as well as a temporal visual region that could include more than one area. The other four superorders emerged later in mammalian evolution from the third major branch of mammals, the eutherian or placental mammals. The Afrotherian superorder is nicely represented by tenrecs from Madagascar. Because tenrecs have a primitive body morphology that is similar to those of members of the order Insectivora, tenrecs were formerly classified as Insectivores. Modern studies put tenrecs squarely in the new superorder of Afrotheria. Tenrecs have little neocortex, as in early mammals, with much of their cortex occupied by primary and secondary sensory areas [27]. A primary motor cortex is suggested by architectonic traits, and its existence is supported by evidence for primary motor cortex in other Afrotherian mammals. Members of the Xenatherian superorder, armadillos, sloths, and anteaters, have not been extensively studied, but the limited evidence that exists indicates that they have at least primary visual, somatosensory, and auditory areas [37]. The superorder, Laurasiatheria, with carnivores, pangolins, bats and core insectivores is well represented by hedgehogs, shrews and moles. In these insectivores, we find evidence for V1, V2, A1, S1, SC, SR, PV, S2, and M1 [4]. Of course, there is evidence for all these areas, and more, in the well-studied domestic cats [14,29]. The remaining superorder, Euarchontoglires, contains humans and other primates, as well as rodents, lagomorphs, tree shrews and flying lemurs. Members of this superorder also have the primary and secondary sensory fields and M1.
The point of this necessarily superficial review of cortical organization in members of the major branches of mammalian evolution is that it is possible to reconstruct the probable areal organization of the neocortex of the first mammals from the distribution patterns of those cortical areas that can be reliably identified in extant mammals. In addition to the sensory areas discussed above, we can infer from such data that several subdivisions of cingulate cortex, with inputs from the anterior group of nuclei of the thalamus, as well as perirhinal cortex, and several divisions of frontal cortex existed in early mammals. Additionally, a visual-limbic area on the medial margin of V1, sometimes called prostriata, was likely to have been present [35]. We can also conclude from comparative studies that the brains of early mammals were small relative to body sizes, which were also small, and proportionally little of the forebrain was devoted to neocortex. This ancestral neocortex was poorly differentiated into layers and neuron types, and it included few cortical areas, on the order of 20 or so [19]. This primitive organization was expanded and differentiated to eventually form the huge and complex human brains with possibly over 200 cortical areas, a tenfold increase in number of areas and a much greater increase in the complexity of the connectional networks of cortical areas.
Tree Shrews and other members of the Euarchontoglires clade
Humans and other primates form one major branch of the Euarchontoglire clade. Another branch, the Glires, includes lagomorphs (rabbits, hares, and pikas) and rodents. It is somewhat disconcerting to realize that these mammals are our rather close relatives, as there is nothing particularly remarkable about their brains. Rabbits have emphasized vision in order to detect and flee from predators. Thus, their temporal cortex is expanded, while frontal cortex remains rather small. Rodents often depend on using their facial whiskers for exploring the environment, but some rodents such as squirrels, have excellent vision, and an expanded and well-differentiated V1 and V2, as well as two or more large visual areas in the temporal lobe [16]. Rodents vary in body size by 1000-fold, from small mice to dog-sized capybaras. While cortical organization has been extensively studied in mice and rats, much less is known about other rodents. Although rodents vary in body and brain size [11], results from the few published studies suggest that rodents do not vary much in numbers of cortical areas, having only a few more than those postulated for early mammals (Fig. 1A). For comparison with tree shrews and primates, where vision is greatly emphasized, the brains of squirrels are useful, but the similarities in cortical organization that exist (e.g., large, well-differentiated V1 and V2) reflect parallel evolution as vision became more important in each group.
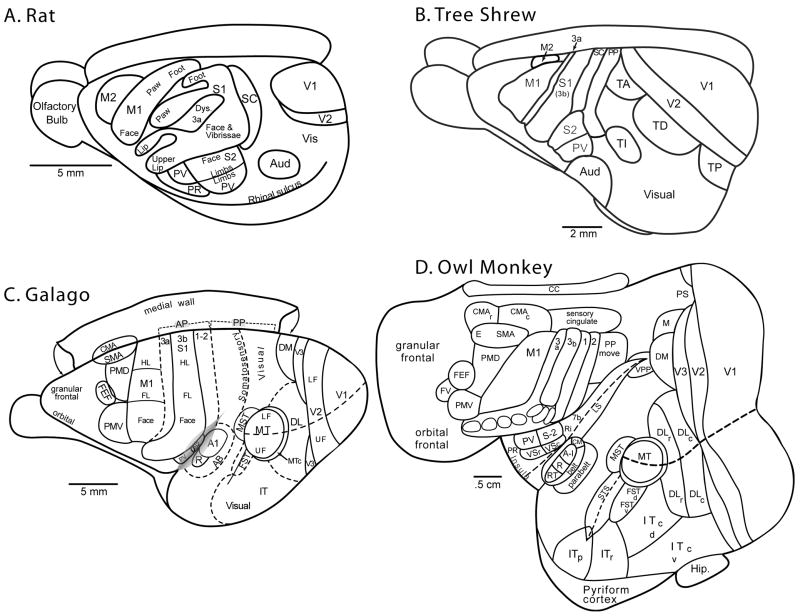
Figure 1.
Some of the cortical areas of (A) rats, (B) tree shrews, (C) galagos, and (D) owl monkeys. The owl monkey cortex has been flattened so that areas in the lateral fissure and on the medial wall can be shown. Rats, like many other small-brained mammals have few cortical areas, including primary visual (V1), auditory (Aud or A1), somatosensory (S1) and motor (M1) areas. Other somatosensory areas include a caudal somatosensory belt (SC) a distorted rostral belt (RC or dys. for dysgranular cortex), a second area (S2), a parietal ventral area (PV) and a parietal rostral area (PR). Motor cortex includes a dorsal premotor area (M2), and visual cortex includes a second visual area (V2) and other visual areas (Visual). Some of the somatotopy of S1, PV, S2, and M1 is indicated. Tree shrews have an expanded temporal lobe with visual functions. Several temporal areas (TP, posterior; TD, dorsal; TA, anterior, and TI, inferior) have been identified, but homologous areas in other mammals are unknown. TA and adjacent visual cortex relay to motor cortex, as posterior parietal (PP) cortex does. Prosimian galagos have more visual cortex, and many of the areas recognized in anthropoid primates (the dorsolateral area, DL or V4; V3; the dorsomedial area, DM; the middle temporal area, MT; the middle superiortemporal area MST; the fundal area of the superior temporal sulcus, FST; and inferior temporal cortex, IT. Posterior parietal (PP) cortex includes a caudal sector with dense visual inputs and projections to a rostral sector with somatosensory inputs and projections to motor and premotor cortex. Motor cortex includes a primary area (M1), dorsal premotor cortex (PMD), ventral premotor cortex (PMV), the frontal eye field (FEF), a supplementary motor area (SMA), and two or more cingulate motor areas (CMA). Hindlimb (HL) and forelimb (FL) portions of 3b and M1 are indicated. Owl monkeys have even more visual cortex, while sharing a number of areas with galagos. The dorsolateral area is divided into rostral (DLr) and caudal (DLc) sectors and inferior temporal cortex has been divided into polar (ITp), rostral (ITr) caudodorsal (ITcd) and caudoventral (ITcv) sectors. FST has dorsal (d) and ventral (v) areas. A medial area (M) and a ventral posterior parietal area (VPP) have been identified. The location of the prostriata area (PS), which may exist in most mammals, is indicated. Anterior parietal cortex contains areas 3a, 3b, 1 and 2, and somatosensory cortex of the lateral sulcus (LS) has rostral (VSr) and caudal (VSc) ventral somatosensory areas, a parietal rostral area (PR), and a retroinsular (Ri) region. Auditory cortex includes A1, a rostral area (R), a rostrotemporal area (RT), a caudomedial area (CM) and other subdivisions of the auditory belt and parabelt. An eye movement (E) region of SMA and a frontal visual region (FV) are labeled. CC, corpus callosum; STS, superior temporal sulcus; hip, hippocampus.
Other Euarchontoglires (the Euarchontans) are the flying lemurs, tree shrews and primates. We know little about the brains of flying lemurs (misnamed because they do not fly, but glide, and they are not lemurs), but histological studies indicate that they have a well-differentiated visual system and probably several visual areas in the expanded temporal cortex, in addition to a large V1 and V2 in occipital cortex [35]. However, tree shrews from Southeast Asia have long been bred in captivity and studied, especially their visual system, as they are diurnal and are highly visual [16,29]. In tree shrews, V1 and V2 are large and structurally well differentiated (Fig. 1B). The visual thalamus is also large, and the lateral geniculate nucleus is laminated. As in squirrels, the superior colliculus is large. Somatosensory cortex includes S1, S2, PV, SC and SR of other mammals [34]. The representation of the forepaw is enlarged in S1, and the forepaw is used for manipulating objects. Frontal cortex includes a large primary motor area and a dorsomedial premotor area. The rest of frontal cortex is proportionally small. Auditory cortex includes A1 and several other fields. Most notably, posterior parietal cortex includes a rostral portion that is predominately somatosensory and has strong connections with motor cortex, and a caudal portion that is predominantly visual with weaker connections with motor cortex.
When we compare the cortical organization of tree shrews (Fig. 1B) with that of primates (Fig. 1C & 1D), we see a number of similarities. Given the organization in tree shrews and the more elaborate organization in primates, it is likely that the immediate ancestors of primates, in comparison to early mammals, had (1) an expanded visual system with more visual areas in an enlarged temporal lobe; (2) an expanded posterior parietal region with visual and somatosensory inputs, and projections to motor and premotor cortex; (3) an enlarged primary motor cortex with more cortex devoted to the forepaw, and (4) subdivisions of anterior parietal cortex that anatomically and physiologically closely resembled areas 3a, 3b (S1), and 1 of primates.
Early primates
Early primates were small, arboreal, and nocturnal. They fed on small insect and vertebrate prey, buds, and fruit [36]. Their brains were moderately expanded, and not much different in size in proportion to body size than the brains of extant prosimian primates (lemurs, lorises, and galagos). Their eyes were large, and frontally directed, and their temporal cortex was enlarged. Thus, vision was obviously important, and adaptations for life in the fine branches of trees suggested that their neural systems for eye-hand coordination were well developed to subserve reaching for food items while clinging to unstable branches [3]. This supposition is born out by the results of comparative studies of brain organization of extant primates. The results of studies of the brains of prosimian primates are especially relevant, as these brains approximate, in size and shape at least, those of early primates.
Much of what is known about brain organization in prosimians comes from studies of African galagos, especially the greater galago (Otolemur garnetti). Visual cortex in these primates (Fig. 1C) includes areas common to all or most mammals (V1 and V2) and areas thought to be unique to primates (MT, MTc, MST, FSTd, FSTv, DM, ITc, ITr) [18]. Some of these areas, those considered to be parts of the dorsal stream of visual processing for visuomotor control [21,42], project directly or indirectly to the caudal half of an enlarged posterior parietal cortex. This posterior parietal region projects in turn to the rostral half of posterior parietal cortex, which also receives somatosensory inputs from higher order somatosensory areas, and sends feedforward projections to premotor areas, which project to primary motor cortex. Electrical stimulation of the rostral half of posterior parietal cortex with microelectrodes reveals a number of subregions, possibly cortical areas, where behaviorally meaningful movements are evoked [39]. These movements include reaching and grasping, as for an object, and hand to mouth retrieving movements. Thus, parts of rostral posterior parietal cortex appear to be specialized for using somatosensory and visual information to help mediate eye-hand coordination for specific types of behavior. The disruption of neural activity in primary motor cortex blocks these evoked behaviors, indicating that M1 is an important node in the circuits that organizes these behaviors. In conclusion, prosimians have brain networks for the kinds of visuomotor guidance of hand use that has been considered to be essential for early primates to occupy their arboreal feeding niche. The presence of a similar network, one that appears to be even more elaborate in anthropoid primates, suggests that the important components of the network were already present in early primates, and were retained in both prosimian and anthropoid lines of decent.
The frontal motor regions, as parts of a sensorimotor network, were enlarged and subdivided in early primates. Rodents and tree shrews have a primary motor area, M1, and a second area, which probably corresponds to dorsal premotor cortex of primates [33]. In contrast, prosimian galagos (Fig. 1C), as well as anthropoid primates (Fig. 1D), have an enlarged M1 with an expanded portion devoted to forelimb and digit movements. Galagos also have dorsal and premotor areas, a frontal eye field for eye movements, a supplementary motor area, and at least two motor areas in cingulate cortex of the medial wall of the cerebral cortex [46]. As these areas are present in both prosimian and anthropoid primates, they likely evolved after the divergence of tree shrews and primates from their common ancestor. Such comparative studies also indicate that early primates had a region of granular frontal cortex with sensory inputs and connections with dorsal and ventral premotor areas, which projected in turn, to M1. Several of these motor areas, together with somatosensory areas, projected to interneurons and motor neurons in the spinal cord, but most of these projections were from M1.
Anterior and lateral somatosensory corticies did not change as dramatically as motor cortex did in early primates, although the rostral somatosensory area closely resembled area 3a of anthropoid primates (see below). Parts of primary somatosensory cortex (area 3b) and adjoining bands of cortex (SR or 3a; SC or 1–2) that represent the tongue might have been involved in taste, together with some of the lateral somatosensory cortex [23]. Auditory cortex included a primary (A1) and a rostral area (R), as well as the surrounding auditory belt of other primates [20].
Altogether, early primates had changed the most from their rodent and tree shrew relatives in having more and larger visual areas, an expanded posterior parietal region with somatosensory and visual subregions, a constellation of motor areas in frontal cortex, and granular prefrontal cortex with sensory inputs and outputs to premotor cortex. These innovations greatly enhanced the use of sensory information in guiding and controlling reaching and other motor behaviors.
Early Anthropoid primates
Anthropoid primates include monkeys, apes and humans. They also include a rather restricted radiation that lead to present-day tarsiers, which have become nocturnal, visual predators of small prey. Tarsiers have become so specialized that they eat no vegetable food. Little is known about how their brains are organized, except that primary visual cortex is huge relative to other brain areas, and much of caudal neocortex is visual [5]. Primary auditory, somatosensory and motor systems also appear to be present.
Cortical organization varies in monkeys (simians). The small marmosets (New World monkeys) have an anterior parietal cortex that resembles that of prosimians in that SR (3a) and SC (area 1) are poorly differentiated [26], but areas 3a, 1 and 2 are well differentiated in the larger New World Cebus monkeys [9]. Thus, there is evidence of parallel evolution of the somatosensory cortex in simians. Also, posterior parietal cortex is large, more subdivided, and more responsive to somatosensory stimuli in the larger New World and Old World monkeys than in marmosets. As an unusual specialization, some of the New World monkeys (cebus, spider) evolved the tip of the tail as a tactile, grasping organ densely packed with cutaneous receptors that activate a large portion of somatosensory cortex [9]. Monkeys also have more visual cortex than prosimians (Fig. 1D), and more clearly defined subdivisions of posterior parietal cortex. In Old World monkeys, dorsal and ventral premotor regions contain two or more fields.
Apes and hominins
Early apes had larger brains than monkeys, but not much can be inferred about the organization of early ape brains since comparative studies of extant ape brains has been so limited. Also, we know only brain sizes and shapes from our extinct hominin relatives (all species more closely related to modern humans than apes) [38]. From modern non-invasive brain imaging studies and from histological studies, an understanding of the organization of the human brain is starting to emerge, although information is limited. However, the evolution of areas devoted to language [7], visual recognition of faces and objects [12], and hemispheric specialization and asymmetries [6] are among the prominent additions to human brains. What is most obvious, however, is the great growth of the brain relative to body size from early ape ancestors to humans [22]. Even present day chimpanzees of about the same body size as humans, and gorillas of considerably larger sizes than humans, have brains of the 300–400 cm3 size, about the size of the brains of early hominins of the genus Australopithecus living some three million years ago. Over the last two million years, the brains of our ancestors increased rapidly to their current 1300–1500 cm3 size.
Such great increases in brain size imply great increases in number of neurons and therefore computational power. Human brains might have as many as 100 billion neurons, compared to 6.4 billion in macaque monkeys [10]. In contrast to rodents (and likely other lines of mammalian evolution), where average neuronal size increased and neuronal densities decreased as larger brains evolved, average neuron size and neuronal densities are rather constant across a great range of brain sizes in primates. Thus, human brains have more neurons for their large size than would be expected for a hypothetical rodent with such a large brain. The evolution of large brains in apes and early hominins, and even larger brains in our recent ancestors and in modern humans, resulted in great increases in the number of neurons. This made it difficult for neurons to interconnect and function in the same ways that were possible in the smaller brains of our ancestors [15]. As brains get more neurons, individual neurons become interconnected with proportionally fewer of the total number of neurons, and the brain becomes more modular so that connections and connection distances do not increase to unworkable extents. As one example, functions in the large human brain became more localized to the right or left cerebral hemisphere so that the need for long and fast interhemispheric connections became reduced. The evidence for more modular organization in the larger primate brains is less complete, but the large primary areas stopped increasing in size, implying a great increase in the numbers of smaller cortical areas. In support of this inference, accumulated evidence from functional imaging studies suggests that human brains have a large number (200 or so) of cortical areas.
In summary, many of the changes that occurred in the evolution of the large human brain remain to be discovered, but much progress is being made in understanding the specific types of modifications that were made in brains during the course of mammalian evolution. As brains became large, scaling problems likely forced modifications in brain organization that included (1) more cortical areas and more modules or types of functional columns within areas; (2) an emphasis on local cortical connections over long connections; and (3) a stabilization of the sizes of the largest cortical areas, as area size impacts on the types of functions that areas best mediate. Small areas are more fully interconnected, and are optimal for global comparisons, while large sensory areas are optimal for comparisons of local detail.
Footnotes
Prepared for a special issue of Brain Research Bulletin on the Proceedings of the 5th ECCN Conference, April 25-28, 2007
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albert JS. Phylogenetic character reconstruction. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 1. Elsevier; London: 2007. pp. 41–53. [Google Scholar]
- 2.Beck PD, Pospichal MW, Kaas JH. Topography, architecture, and connections of somatosensory cortex in opossums: evidence for five somatosensory areas. J Comp Neurol. 1996;366:109–133. doi: 10.1002/(SICI)1096-9861(19960226)366:1<109::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Block JI, Boyer DM. Grasping primate origins. Science. 2002;298:1606–1610. doi: 10.1126/science.1078249. [DOI] [PubMed] [Google Scholar]
- 4.Catania KC, Collins CE, Kaas JH. Organization of sensory cortex in the East African Hedge Hog (Atelerix albiventris) J Comp Neurol. 2000;421:256–274. doi: 10.1002/(sici)1096-9861(20000529)421:2<256::aid-cne10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Collins CE, Hendrickson A, Kaas JH. Overview of the visual system of tarsius. Anat Rec. 2005;287A:1013–1025. doi: 10.1002/ar.a.20263. [DOI] [PubMed] [Google Scholar]
- 6.Corballis MC. Evolution of hemispheric specialization of the human brain. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 4. Elsevier; London: 2007. pp. 379–393. [Google Scholar]
- 7.Deacon TW. The evolution of language systems in the human brain. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 4. Elsevier; London: 2007. pp. 529–548. [Google Scholar]
- 8.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 9.Felleman DJ, Nelson RJ, Sur M, Kaas JH. Representations of the body surface in areas 3b and 1 of postcentral parietal cortex of cebus monkeys. Brain Res. 1983;268:15–26. doi: 10.1016/0006-8993(83)90386-4. [DOI] [PubMed] [Google Scholar]
- 10.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci USA. 2006;103:12138–12143. doi: 10.1073/pnas.0604911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman KL, Gauthier I. Evolutionary specializations for processing faces and objects. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 4. Elsevier; London: 2007. pp. 437–445. [Google Scholar]
- 13.Jerison HJ. What fossils tell us about the evolution of the neocortex. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 3. Elsevier; London: 2007. pp. 1–12. [Google Scholar]
- 14.Kaas JH. The segregation of function in the nervous system: why do sensory systems have so many subdivisions? In: Neff WP, editor. Contributions to Sensory Physiology. Academic Press; New York: 1982. pp. 201–240. [Google Scholar]
- 15.Kaas JH. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain and Mind. 2000;1:7–23. [Google Scholar]
- 16.Kaas JH. Convergences in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behav Evol. 2002;59:262–272. doi: 10.1159/000063563. [DOI] [PubMed] [Google Scholar]
- 17.Kaas JH. Evolution of somatosensory and motor cortex in primates. Anat Rec. 2004;281A:1148–1156. doi: 10.1002/ar.a.20120. [DOI] [PubMed] [Google Scholar]
- 18.Kaas JH. The evolution of sensory and motor systems in primates. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 4. Elsevier; London: 2007. pp. 35–57. [Google Scholar]
- 19.Kaas JH. Reconstructing the organization of the forebrain of the first mammals. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 3. Elsevier; London: 2007. pp. 27–48. [Google Scholar]
- 20.Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.02.008. PMID: 17433837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaas JH, Preuss TM. Human brain evolution. In: Squire LR, editor. Fundamental Neuroscience. Vol. 2. Academic Press; San Diego: 2003. pp. 1147–1166. [Google Scholar]
- 23.Kaas JH, Qi HX, Iyengar S. Cortical network for representing the teeth and tongue in primates. Anat Rec. 2006;288A:182–190. doi: 10.1002/ar.a.20267. [DOI] [PubMed] [Google Scholar]
- 24.Karlen SJ, Krubitzer LA. The functional and anatomical organization of marsupial neocortex: evidence for parallel evolution across mammals. Prog in Neurobiol. 2007;82:122–141. doi: 10.1016/j.pneurobio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krubitzer LA. What can monotremes tell us about brain evolution? Phil Trans B Roy Soc London. 1998;353:1127–1146. doi: 10.1098/rstb.1998.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 1990;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krubitzer LA, Künzle H, Kaas JH. Organization of sensory cortex in a Madagascan insectivore, the tenrec (Echinops telfairi) J Comp Neurol. 1997;379:399–414. [PubMed] [Google Scholar]
- 28.Luppino G, Rizzolatti G. The organization of the frontal motor cortex. News Physiol Sci. 2000;15:219–224. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- 29.Lyon DC. The evolution of visual cortex and visual systems. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 3. Elsevier; London: 2007. pp. 267–306. [Google Scholar]
- 31.Murphy WJ, Eizirik E, O’Brien SJ, Madesen O, Scally M, Douady CJ, Teeling E, Ryder OA, Stanhope MJ, de Jong WW, Springer MS. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science. 2001;294:2348–2351. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- 32.Picard N, Strick PO. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 33.Remple MS, Reed JL, Stepniewska I, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia blangeri): I. Architecture, microelectrode maps and corticospinal connections. J Comp Neurol. 2006;497:133–154. doi: 10.1002/cne.20975. [DOI] [PubMed] [Google Scholar]
- 34.Remple MS, Reed JL, Stepniewska I, Lyon DC, Kaas JH. The organization of frontoparietal cortex in the Tree Shrew (Tupaia belangeri): II Connectional evidence for a frontal-posterior parietal network. J Comp Neuro. 2007;501:121–149. doi: 10.1002/cne.21226. [DOI] [PubMed] [Google Scholar]
- 35.Rosa MG, Krubitzer LA. The evolution of visual cortex: where is V2? TINS. 1999;22:242–248. doi: 10.1016/s0166-2236(99)01398-3. [DOI] [PubMed] [Google Scholar]
- 36.Ross CF, Martin RD. The role of vision in the origin and evolution of primates. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 4. Elsevier; London: 2007. pp. 59–78. [Google Scholar]
- 37.Royce JG, Martin GF, Dom RM. Functional localization and cortical architecture in the nine-banded armadillo (Dasypus novemainetus mexicanus) J Comp Neurol. 1975;164:495–522. doi: 10.1002/cne.901640408. [DOI] [PubMed] [Google Scholar]
- 38.de Sousa A, Wood B. The hominin fossil record and the emergence of the modern human central nervous system. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 4. Elsevier; London: 2007. pp. 291–336. [Google Scholar]
- 39.Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci USA. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Striedter GF. Principles of Brain Evolution. Sinauer Assoc.; Sunderland, MA: 2005. [Google Scholar]
- 41.Ulinski PS. Visual cortex of turtles. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 2. Elsevier; London: 2007. pp. 195–203. [Google Scholar]
- 42.Ungerleider LG, Haxby JV. What and where in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 43.Van Essen DC. Cerebral cortical folding patterns in primates: why they vary and what they signify. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 4. Elsevier; London: 2007. pp. 267–276. [Google Scholar]
- 44.Wicht H, Northcutt RG. The forebrain of the Pacific hagfish: a cladistic reconstruction of the ancestral craniate forebrain. Brain Behav Evol. 1992;40:25–64. doi: 10.1159/000108540. [DOI] [PubMed] [Google Scholar]
- 45.Wiley EO. Phylogenetics: The Theory and Practice of Phylogenetic Systematics. John Wiley & Sons; New York: 1981. [Google Scholar]
- 46.CW-H Wu, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]