Abstract
The majority of bone cell biology focuses on activity on the surface of the bone with little attention paid to the activity that occurs below the surface. However, with recent new discoveries, osteocytes, cells embedded within the mineralized matrix of bone, are becoming the target of intensive investigation. In this article, the distinctions between osteoblasts and their descendants, osteocytes, are reviewed. Osteoblasts are defined as cells that make bone matrix and osteocytes are thought to translate mechanical loading into biochemical signals that affect bone (re)modeling. Osteoblasts and osteocytes should have similarities as would be expected of cells of the same lineage, yet these cells also have distinct differences, particularly in their responses to mechanical loading and utilization of the various biochemical pathways to accomplish their respective functions. For example, the Wnt/β-catenin signaling pathway is now recognized as an important regulator of bone mass and bone cell functions. This pathway is important in osteoblasts for differentiation, proliferation and the synthesis bone matrix, whereas osteocytes appear to use the Wnt/β-catenin pathway to transmit signals of mechanical loading to cells on the bone surface. New emerging evidence suggests that the Wnt/β-catenin pathway in osteocytes may be triggered by crosstalk with the prostaglandin pathway in response to loading which then leads to a decrease in expression of negative regulators of the pathway such as Sost and Dkk1. The study of osteocyte biology is becoming an intense area of research interest and this review will examine some of the recent findings that are reshaping our understanding of bone/bone cell biology.
Keywords: Osteocytes, bone, Wnt/β-catenin signaling, Mechanosensation, Mechanical loading
Osteocytes compose over 90–95% of all bone cells in the adult skeleton and are thought to respond to mechanical strain to send signals of resorption or formation (70). Osteocytes are usually regularly dispersed throughout the mineralized matrix especially in cortical bone. These cells are connected to each other and cells on the bone surface through dendritic processes that occupy tiny canals called canaliculi (For review see (17)). Not only do these cells communicate with each other and with cells on the bone surface but their dendritic processes are in contact with the bone marrow (59) giving them the potential to recruit osteoclast precursors to stimulate bone resorption (133)(12) and to regulate mesenchymal stem cell differentiation (48).
Osteocytes as coordinators of skeletal responses to mechanical loading
In the absence of loading, bone is lost and in the presence of loading, bone is either maintained or increased. The skeleton is unique in its ability to adaptively remodel in response to its perception of mechanical loading or lack of loading or disuse (reviewed in (21)(31)(18). Adaptive remodeling is defined as the constant remodeling of the bone in order to resist and withstand average daily loads. The cells of bone with the potential for sensing mechanical strain and translating these forces into biochemical signals include bone lining cells, osteoblasts and osteocytes. Due to their distribution throughout the bone matrix and extensive interconnectivity, osteocytes are thought to be one if not the major bone cell type responsible for sensing mechanical strain and orchestrating signals of resorption and formation. The osteocyte appears to be capable of relating the intensity of strain signals and the distribution of the strain throughout the whole bone into signals to regulate (re)modeling (70). Recently it has been shown that targeted deletion of osteocytes results in bone loss and bone with targeted deletion of osteocytes does not respond to unloading. These studies show that osteocytes are necessary to maintain bone mass in response to normal load, but in the absence of load send signals of resorption (113).
Role of the Wnt pathway in bone cell function
The Wnt signaling pathway was known to be important in the development and patterning of the skeleton since the early-mid 1990s when studies demonstrated that Wnt-3a mutations resulted in altered mouse axial development (42). A few years later it was shown that the low-density lipoprotein receptor-related protein 6 (Lrp6) knockout (Lrp6-/-) mouse had developmental abnormalities that phenocopied many of the defects observed in mice carrying mutations in Wnt-3a, Wnt-1 or Wnt-7a genes (97). Mutations in human LRP5 were then shown to result in low or high bone mass and clearly demonstrated the importance of the Wnt/β-catenin signaling pathway in the regulation of adult peak bone mass (40)(74)(19).
Wnts are secreted glycoproteins that are also post-translationally modified by the addition of lipid (palmitate), which is required for activity. There are 19 known Wnt proteins (genes) in vertebrates and there are currently 4 different signaling pathways through which any given Wnt protein can act. The best studied of these pathways is often referred to as the canonical pathway, but for clarity sake we will refer to it by its more proper designation as the Wnt/β-catenin signaling pathway. The three other Wnt signaling pathways that have been described in the literature include the planar cell polarity (PCP) pathway (83), the Wnt/Ca+2 pathway (67) and a Protein Kinase A pathway involving CREB (23).
The Wnt/β-catenin pathway is activated by the binding of the appropriate Wnt to a co-receptor complex involving either Lrp5 or Lrp6 (or Arrow in Drosophila) and one of the frizzled family member that belong to the class of 7 transmembrane spanning proteins. Binding to the co-receptor complex leads to the activation of Dishevelled (Dsh) by phosphorylation mediated by frizzled and likely involving the protein kinases casein kinase 1 (95) and 2 (127) and/or PAR-1 (112). Activation of Dsh leads to the downstream phosphorylation of Glycogen Synthase Kinase-3β (GSK-3β) at the serine-9 residue. GSK-3β is a key component of a large “degradation” complex of proteins responsible for controlling free intracellular levels of β-catenin. The alpha isoform (GSK-3α) can also be phosphorylated (at serine-21) and may also play a role in controlling β–catenin levels (80)(7). Normally GSK-3β is responsible for the phosphorylation of β-catenin, which leads to its ubiquitination and degradation by the 26S proteosome complex (1).
Normally the free intracellular levels of β–catenin are kept low by the activity of the above mentioned degradation complex which consists of the scaffold protein axin which binds GSK-3β, the adenomatous polyposis coli (APC) protein, Protein Phosphatase 2A (PP2A), β–catenin and several other proteins (85)(53)(51)(53)(46)(13)(130)(108)(34)(35). Binding of Wnt to Lrp5 and frizzled, in addition to Dsh activation, also results in the phosphorylation of the cytoplasmic tail of Lrp5, likely mediated by Casein kinase 1γ (CK-1γ) (29), the binding of FRAT-1 to this tail and ultimately its association with axin. Axin binding to Lrp5 and the inhibition of GSK-3β results in the collapse of the degradation complex and the release of non-phosphorylated β-catenin into the cytoplasm where is can subsequently translocate into the nucleus to affect gene transcription. Recent studies by Niehrs and colleagues have shown that Wnt induces the coclustering of Lrp6/frizzled and Dsh and this helps to facilitate phosphorylation of the Lrp6 cytoplasmic tail by CK-1γ (15).
Once stabilized β–catenin accumulates in the cytoplasm and then translocates into the nucleus to affect gene transcription by a mechanism that is not well understood. Once in the nucleus β-catenin interacts with the Tcf/Lef family of transcription factors to regulate the expression of a number of genes important to the differentiation, proliferation, apoptosis and functionality of bone cells. Evidence has also been presented for an interaction with the FOXO family of transcription factors that are important in cellular responses to oxidative stress (33)(4). There is a large number of proteins (see http://www.stanford.edu/~rnusse/ for a detailed listing) that participate in the transcriptional regulation initiated by β-catenin. It is currently thought that β-catenin regulates target gene transcription by mediating the formation of a larger complex that induces a change in chromatin structure (11).
In vitro models of mechanical strain
The parameters of in vivo mechanical loading of bone that results in bone formation and/or bone resorption are fairly well established (106)(107). However, how this external loading signal in bone is transmitted at the cellular level is not well established and can be controversial. Clearly, the bone matrix is undergoing tissue deformation, but to what extent and how is not clear. It is also not clear whether this tissue deformation is transmitted to the osteocyte cell membrane through tethering elements causing cell body and/or dendrite deformation or through perturbation of the bone fluid or both (See Figure 1).
Figure 1.
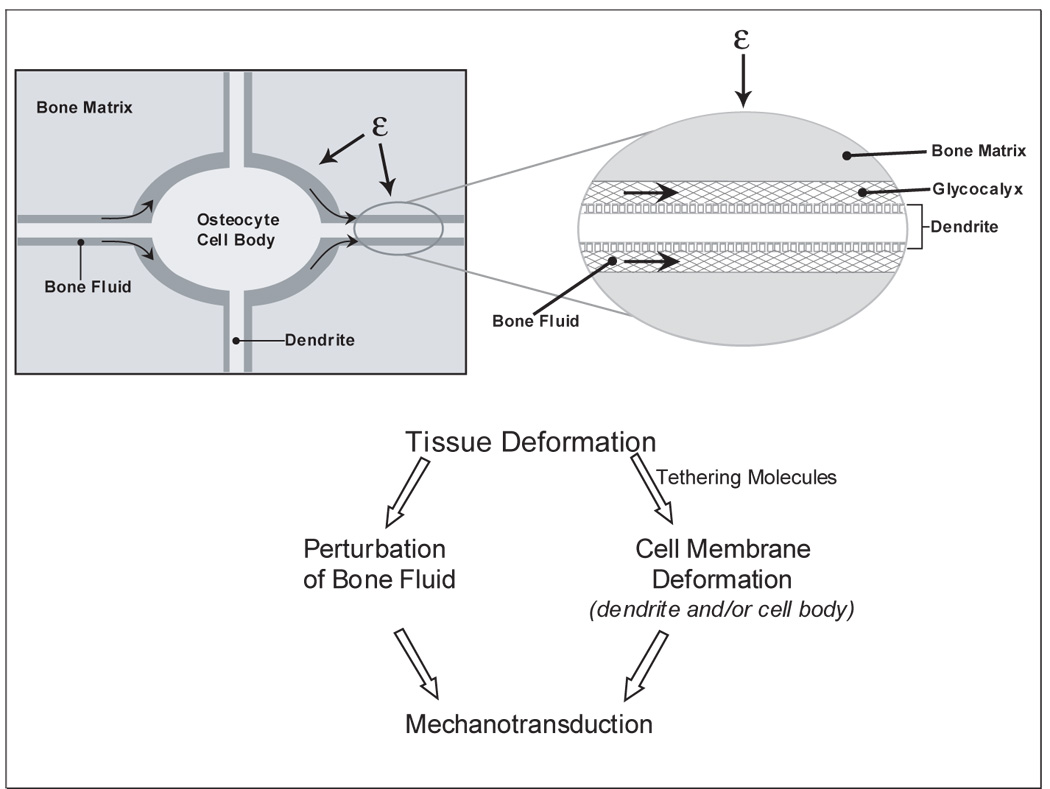
A simplified diagram of the potential effects of tissue deformation on osteocytes. With the application of mechanical loading to the bone, bone matrix is deformed. Deformation of the bone matrix surrounding osteocytes could lead to the perturbation of bone fluid leading to shear stress. Alternatively or in combination with, tissue deformation could lead to perturbation of the cell membrane (whether the cell body or the dendritic processes) through tethering elements thought to be present in the glycocalyx. It is most likely that both contribute to mechanosensation and mechanotransduction.
Tissue Strain, Substrate deformation, Cell deformation
Attempts have been made to correlate the magnitude of bone strain as measured by strain gages to magnitude of either substrate stretching or shear stress on bone cells in cultures with little success. This is most likely due to the fact that bone is inhomogeneous and that strain gages average strain over large areas of bone. It has been shown that at the level of osteocytes, the surrounding bone matrix is heterogeneous resulting in magnified local tissue strains (88)(87). It was shown that application of 2000 microstrain macroscopically to a piece of bone resulted in greater microscopic strain surround osteocyte lacunae of over 30,000 microstrain (87). Osteons, canaliculi, and lacunae are potential stress concentrators (27). It was found that perilacunar tissue strain levels in many lacunae exceed global applied strain.
Recently, it has been shown that the perilacunar matrix surrounding osteocytes can be modified by specific pharmacological treatments such as PTH (114) or glucocorticoids (69). Models taking into account these perilacunar changes showed that maximum strain increases with a decrease in perilacunar tissue modulus and vice versa (100). This model supported previous findings using bone tissue that application of average macroscopic strains similar to strain levels measured in vivo can result in significantly greater perilacunar tissue strains and canalicular deformations.
Fluid Flow Shear Stress
It is generally well accepted that bone fluid flows through the lacuno-canalicular system generating shear stress. Originally steady or laminar fluid shear stress was used in vitro to mimic in vivo shear stress (103)(128), this was followed by pulsatile fluid flow (49), followed by oscillatory flow (54). Several research groups have developed both analytical and numerical models in an attempt to predict fluid flow derived shear stresses in bone (124)(123)(122)(110)(44)(5). However, as with any model, predicted numbers are not absolutely accurate. It is difficult to take into account the number of dendrites per cell, the number of bifurcations per dendrite, and the changes in lacunar and canalicular number with age and the configuration of osteocytes within the lacunar-canalicular system. These parameters are not completely characterized and appear to vary depending on bone type. The predicted levels of shear stress resulting from these models could vary up to 2–3 fold or greater from actual physiological levels. At present, it is proposed that the steady state component of flow is 0.5 to 2 dynes/cm2 and that the alternating component is 8 dynes/cm2 (121). This is based on their theory that transport is due to mechanical loading of the bone and not through pulsatile extravascular pressure (66)(65). They propose that muscle contractions are responsible for basal transport of nutrients to the osteocytes and that stresses due to exercise can be superimposed. These theoretical models are continually being modified based on new information. The limitations of applied in vitro fluid flow shear stress to cell cultures must always be considered when interpreting data or extrapolating back to in vivo conditions.
Movement of cell body and dendritic processes
For decades, the osteocyte has been viewed as an inactive, quiescent cell type and has been referred to as a “placeholder” in bone. However, Dallas and colleagues have shown that osteocyte embedded in bone can extend and retract their dendritic processes and that their cell body undergoes deformation. Calvarial explants from transgenic mice with green fluorescent protein (GFP) expression targeted to osteocytes were used (56) for time lapse dynamic imaging to image living osteocytes within their lacunae (118). Far from being a static cell, the osteocyte was found to be highly dynamic. Osteocyte dendrites, rather than being permanent connections between osteocytes and between osteocytes and surface cells, may be capable of redirecting signaling between cells similar to the “switchboard” principle. Giving the evidence for the motility of osteocyte dendrites, the models involving tethering in mechanosensation may need revisiting because these tethers would have to either be transient or constantly being broken and remade through some active process.
The potential role of cilia in bone cell mechanotransduction
Single cilia are present on primary bone cells and osteoblast and osteocyte cell lines and PKD1 and PKD 2, components of cilia with known mechanosensory proteins in the kidney, do play a role in normal bone structure (129). It remains to be determined if PKD1 and PKD2 function in a similar manner in both kidney and bone (86). Recently, Jacobs and colleagues have shown that primary cilia in bone cells do not mediate calcium flux in response to fluid flow, therefore the mechanism used by cilia in bone cells is distinct from that of kidney cells (76). Reducing the number of cilia reduces the induction of osteopontin in MC3T3 osteoblast cells, the induction of prostaglandin in both MC3T3 and MLOY4 osteocyte cells, and the increase of COX2 and OPG/RANKL ratio in MLOY4 cells in response to fluid flow shear stress. These authors suggest that regulation of the OPG/RANKL ratio is one means by which formation and resorption can be maintained in equilibrium.
Osteocytes are not Osteoblasts
The differences between markers, morphology, and function of the polygonal matrix producing osteoblast and the embedded, dendritic osteocyte are dramatic, yet the practice of using osteoblast cell lines to represent osteocytes continues. The osteoblast is unlikely to be subjected to fluid flow shear stress in vivo and if so, the form and magnitude would be distinctly different from what occurs in the osteocyte lacunocanalicular system. The osteoblast may be more likely to be subjected to tissue deformation as represented by substrate stretching. The polygonal shape of the osteoblast would respond differently and result in significant differences with regards to cell deformation compared to an osteocyte. Also, the osteoblast lacks dendritic processes that may play a significant role in mechanosensation (117) and osteoblasts make up under 5% of the total number of cells on the bone surface in the adult animal.
All eukaryotic cells in general are sensitive to mechanical forces (94). For many years, it was acceptable to use osteoblast cell lines as surrogates for osteocytes due to difficulties in isolating these cells from bone and the absence of osteocyte-like cell lines. Isolation of primary osteocytes is difficult yielding small numbers and a heterogeneous population. Regardless of how difficult or tedious the isolation, several investigators used primary osteoblasts and osteocytes to show significant differences in markers, function, sensitivity and response to mechanical strain (89)(90)(81)(82). For example, it has been shown the primary osteoblasts are less sensitive than primary osteocytes to shear stress (39)(64) that shear stress induced less of a calcium response in a single primary osteocyte than a single primary osteoblast,(60), that shear stress induced prostaglandin production in osteocytes is inhibited by cyoskeletal disruption, but not in osteoblasts (79), different ion channels are involved in osteoblast as compared to osteocytes’ response to strain (101), stress fiber organization is delayed in osteocytes compared to osteoblast-like cells in response to fluid flow (98), and osteocytes have a gene expression profile distinct from osteoblasts (45).
In spite of this data, many investigators continue to use osteoblast cell lines to study mechanosensation, mainly because of availability and ease of use. The introduction of the osteocyte-like MLO-Y4 cell line has allowed investigators access to a cell line with properties of the osteocyte (61). Like primary osteocytes, this cell line is extremely sensitive to shear stress (96), has a dendritic morphology, expresses high amounts of CD44 and Cx43 similar to primary osteocytes (50)(43), and high amounts of E11/gp38, a marker of the early osteocyte (132). Like avian osteocytes, this cell line will support osteoclast formation in the absence of any osteotropic factors (133) and also when damaged in 3D culture will support osteoclast formation (68). This cell line has been shown by numerous investigators to also support not only osteoblast function such as differentiation and matrix formation, but also support mesenchymal stem cell differentiation (48). The field would benefit from the generation of a cell line with the properties and function of the mature osteocyte.
The role of Wnt signaling in response to load
The original analysis from studies of the High Bone Mass kindred suggested that affected family members had an inappropriate bone formation response to normal physiologic loads (55) as if the bone “mechanostat” (36) had been set differently. The HBM transgenic mice that carry the LRPG171V cDNA driven by the 3.6kb Col1A1 promoter have been shown to recapitulate the phenotype observed in the skeleton of the affected members of the kindred (8) and interestingly it was noted that these mice had decreased osteoblast and osteocyte apoptosis. This result is consistent with not only building a denser skeleton (longer-lived osteoblasts), but also having made an appropriate adjustment to maintain the increased bone density (longer-lived osteocytes). These findings implied an important role for Lrp5 and the Wnt/β-catenin signaling pathway relating to osteocyte/osteoblast apoptosis.
The Wnt pathway clearly plays an important role in bone cell differentiation, proliferation and apoptosis as shown by a number of studies (reviewed in (126)(47)(16)). Recent work has further demonstrated that Lrp5 and the Wnt/β-catenin signaling pathway are absolutely required for bone formation in response to mechanical loading (55)(109)(104)(71). Wnt induces the coclustering of Lrp6/frizzled and Dsh called “signalsomes” that facilitate phosphorylation of the Lrp6 cytoplasmic tail by CK-1γ (15).These “signalsomes” are partially positive for Caveolin, which Rubin and colleagues have shown may play a role in regulating cytoplasmic β-catenin levels in response to mechanical load/strain in osteoblasts (75). Some recent data presented by Lanyon and co-workers (6) suggests that the estrogen receptor alpha isoform (ER-α) may play a role in shuttling β-catenin into the nucleus in response to mechanical strain in osteoblasts. This may in part explain how estrogen regulates bone mass through a functional intersection through ER-α with Wnt/β–catenin signaling.
Regulation of the Wnt/β-catenin signaling pathway is vested largely in proteins that either act as competitive binders of Wnts, notably the sFRP family of proteins, or act at the level of Lrp5; including the osteocyte specific protein, sclerostin (the Sost gene product) (73)(32), Wise (52) and the Dickkopf (Dkk) proteins, particularly Dkk-1 and Dkk-2 (20)(14)(72). Sclerostin has been shown to be made by mature osteocytes and inhibits Wnt/β-catenin signaling by binding to Lrp5 and preventing the binding of Wnt (116)(99)(32). Dkk-1 though expressed in many cell types is highly expressed in osteocytes (72). Dkk1 has been shown to also bind to Lrp5 and in studies with the various mutations in Lrp5 that give rise to high bone mass it has been shown that these mutations reduce the ability of Dkk-1 to bind Lrp5 (3). Dkk-1 inhibits Wnt activation of the pathway by binding to Lrp5/6 and a transmembrane protein, Kremen. The tertiary complex that is formed is internalized and thus lrp5 is removed from the cell surface and unable to bind Wnt (78). Mutations in Sost and Dkk-1 in humans and/or mice have been shown to result in increased bone mass (10)(9)(111)(84) consistent with a role of the Wnt/β-catenin signaling pathway in bone mass regulation. Clinical trial studies using antibodies to sclerostin have also been shown to result in increased bone mass, suggesting that targeting of these negative regulators of Wnt/β-catenin signaling pathway might be anabolic treatments for diseases such as osteoporosis (93)(92). Finally, mechanical loading has been shown to reduce sclerostin levels in bone (105) suggesting that one of the targets of the pathways that are activated by the early events after mechanical loading are the genes encoding these negative modulators of the Wnt/β-catenin signaling pathway. Downregulation of these genes and reduced protein levels could create a permissive environment in which Wnt proteins that might be present or whose expression was induced by loading could then activate the pathway.
Wnt signaling in osteocytes, crosstalk with the prostaglandin pathway
Considerable progress has been made in terms of understanding the role of Wnt/β-catenin signaling in osteogenesis and particularly in osteoblast biology, however much less in known about the function of this pathway in the osteocyte. However, new data is emerging (much of which is currently only been published in abstract form) that identifies the Wnt/β-catenin pathway as a critical regulator of osteocyte biology, particularly as relates to bone responsiveness to mechanical loading.
Fluid flow-induced shear stress stimulates gap junction-mediated intercellular communication and increases Cx43 expression by the release of prostaglandin (25). PGE2 in turn functions in an autocrine fashion to activate EP2 receptor signaling, including increased intracellular cAMP and activated PKA (24). Connexins not only form gap junctions but can function as un-apposed halves of gap junction channels called hemichannels, not requiring physical contact with adjacent cells (41). Not only osteocytes but also neural progenitors and neurons, astrocytes, heart, and osteoblasts have functional hemichannels formed by Cx43. The opening of hemichannels appears to provide a mechanism for ATP and NAD+ release, which raises intracellular Ca2+ levels . Hemichannels expressed in MLO-Y4 cells function as transducers of the antiapoptotic effects of bisphosphonates (38) and directly serve as a portal or pathway for the exit of intracellular PGE2 in osteocytes induced by fluid flow shear stress (26). Therefore, the release of prostaglandin in response to shear stress through hemichannels has an autocrine effect on osteocytes through the EP2 receptor.
Castellone et al (22) have shown that the PGE2 mediated group of colon cancer cells was through a Wnt/β-catenin pathway crosstalk mechanism. The mechanism they put forward involved binding of PGE2 to its EP2 receptor and then the activation of the trimeric G-protein associated with this receptor. The Gβγ subunits activated PI3-kinase, which in turn activated Akt, which then phosphorylated GSK-3β, thereby inhibiting its phosphorylation of β-catenin. At the same time the Gα subunit bound to Axin and promoted dissociation of the β-catenin degradation complex. The net result of this concerted action of these G-proteins was to activate β-catenin signaling, independent of Wnt binding to the Lrp5/frizzled co-receptor complex.
We have recently shown that mechanical loading of the ulna of the TOPGAL mouse (28), which carries a LacZ reporter gene driven by three Tcf consensus promoter sequences, results in activation of β-catenin signaling within 1 hour after a single loading session that was only detectable in the osteocytes. By 24 hours post loading we observed evidence of Wnt/β-catenin signaling activation in cells on the bone surfaces, suggesting that a signal produced by the osteocyte was being propagated to surrounding osteocytes and eventually to the bone surface. The nature of this signal is unknown at present. These data support the concept that the osteocyte is the primary sensory cell detecting mechanical loading in bone. In an effort to understand how β-catenin is so rapidly activated in the osteocyte in response to mechanical loading, we have recently demonstrated that PGE2 and fluid flow shear stress treatment of MLO-Y4 osteocytes results in increased phosphorylation of GSK-3β, β-catenin nuclear translocation and changes in the expression of β-catenin target genes (57)(58). The release of PGE2 by bone in response to mechanical loading has long been known to be one of the earliest responses to loading (102). Furthermore, Turner and colleagues (109) have shown that when they applied mechanical load to Lrp5-/- mice a normal PGE2 release occurred even though no bone formation occurred. Their data imply that PGE2 release occurs both before and independent of Lrp5 and Wnt/β-catenin signaling. Increased phosphorylation of Akt has also been demonstrated in response to mechanical loading of osteoblasts (91).
Other signaling pathways that are important in response mechanical loading may also crosstalk with the Wnt/β–catenin signaling pathway. For example activation of integrins leading to stimulation of integrin-linked kinase, which can phosphorylate GSK- 3β is another intersection that needs to be explored (30) and potentially any pathway that activates Akt could crosstalk with the Wnt/β-catenin pathway (37). The estrogen/estrogen-receptor axis may also prove an important intersection (6). Furthermore, with respect to PGE2, it remains uncertain as to what upstream events control its release and all of these will need to be understood before we have an integrated understanding of how osteocytes perceive mechanical loading and translate this into biochemical signals that ultimately alter the behavior of various bone cells to produce their response. Also, the potential involvement of the other Wnt signaling pathways (83)(67)(23) in osteocyte biology or osteocyte responses to mechanical loading is not presently understood. Examination of other Wnt pathways in osteocyte function is warranted given that fluid flow has been shown to induce Cox-2 expression in MC3T3-E1 osteoblasts via a PKA signaling pathway (120)(119) and PKA can be a downstream target of non-canonical Wnts (23). The BMP signaling pathway and the Wnt/β-catenin pathway appear to crosstalk as has been described in osteoblast biology. It has been shown that the inhibitory action of sclerostin on BMP-stimulated bone formation is due to sclerostin binding to Lrp5/6, thereby inhibiting Wnt/β-catenin signaling (115). Whether these two pathways interact solely at the level of the osteoblast or may also function in osteocytes is not known. Thus there are a number of pathways that may be involved in the complex regulation of osteocyte responses to mechanical loading. Identifying and understanding how these various pathways interact is a major challenge, even more so in the osteocyte.
The role of the Wnt pathway in preventing osteocyte apoptosis
Increased activity of the Wnt/β-catenin signaling pathway is well known to be associated with decreased apoptosis (2) and in many cases is associated with the development of cancers (131). We have further examined the role of the Wnt/β-catenin signaling pathway in bone with regard to dexamethasone induced apoptosis of osteocytes, which has been postulated to be the major mechanism underlying glucocorticoid mediated osteoporosis (125)(77). Our recent results demonstrate that mechanical loading of the MLO-Y4 osteocytic cell line can protect against dexamethasone induced apoptosis of these cells (62). The mechanism of this protective effect of mechanical loading appears to be partially mediated through prostaglandin E2 (PGE2) crosstalk with β-catenin signaling (63). Thus in addition to its role in bone responsiveness to mechanical loading, the Wnt/β-catenin signaling pathway also plays an important in osteocyte apoptosis. We have proposed a model (57)(58) (Figure 2) that integrates these various findings into a pathway for the role of PGE2, Lrp5 and Wnt/β-catenin signaling in bone.
Figure 2.
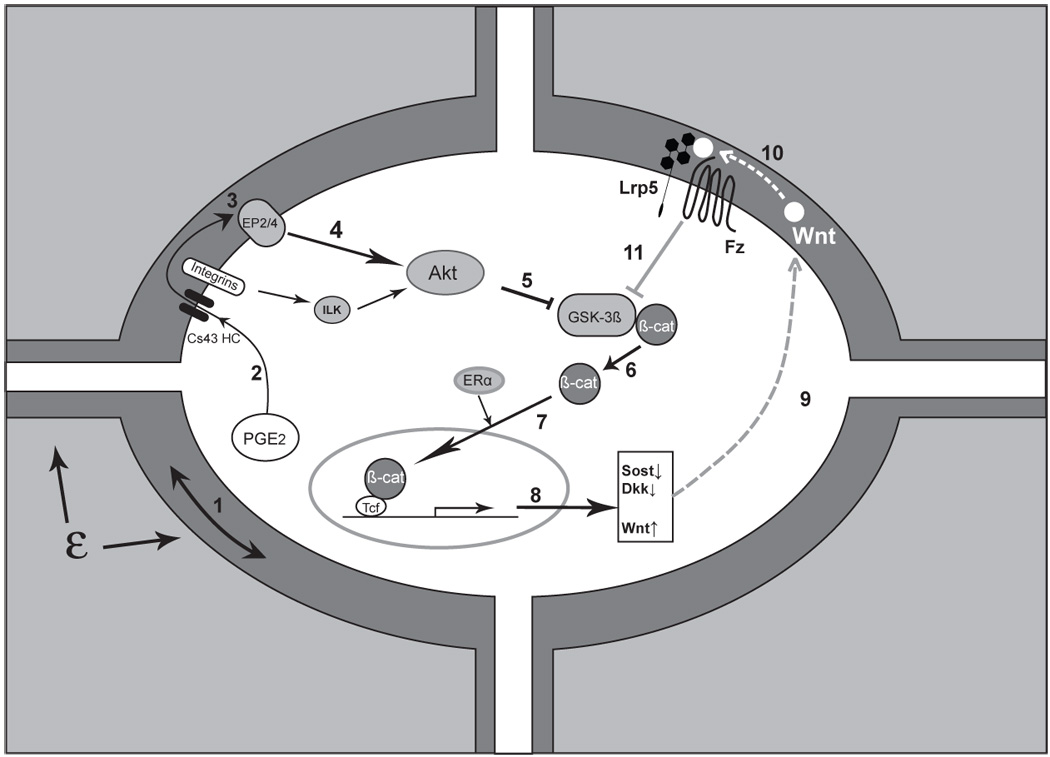
Triggering and amplification of the Wnt/β-catenin pathway in osteocytes in response to load. Mechanical load applied to bone (ε) is perceived by the osteocyte through an unknown mechanism, although fluid flow induced through the lacunarcanalicular system may be a critical component of this perception, ‘step 1’. Perception of load (strain) triggers a number of intracellular responses including the release of PGE2, ‘2’ through a poorly understood mechanism into the lacunar-canalicular fluid where it can act in an autocrine and/or paracrine fashion. Connexin-43 hemichannels (CX43 HC) in this PGE2 and integrin proteins appear to be involved. Binding of PGE2 to its EP2 and/or EP4 receptor, ‘3’, leads to a downstream inhibition of GSK-3β, ‘5’ (likely mediated by Akt, ‘4’) and the intracellular accumulation of free β-catenin, ‘6’. (Integrin activation can also lead to Akt activation and GSK-3β inhibition.) New evidence suggests that ER may participate in the nuclear translocation of β-catenin, ‘7’ which leads to changes in the expression of a number of key target genes ‘8’. One of the apparent consequences is the reduction in sclerostin and Dkk1,’9’ with increased expression of Wnt, ‘10’ (which one or ones is unknown). The net result of these changes is to create a permissive environment for the binding of Wnt to Lrp5-Fz and an amplification of the load signal, ‘11’. (See text for more details and references)
Concluding Comments
The study of osteocyte biology is rapidly becoming an important area of research in the bone field as more and more investigators appreciate the importance of this often overlooked bone cell. There are still many challenges to be overcome. The need to develop more “osteocyte-like” cell lines is clearly one hurdle that confronts this research. There is still much to be learned regarding how the osteocyte senses and transmits signals in response to or absence of loading and orchestrates the activity of other cells. The identity of these signals needs to be determined. Only the “tip of the iceberg” is visible, revealed by the few studies to date. The osteocyte is joining the osteoblast and osteoclast as targets for therapeutics to treat or prevent bone disease. Clearly targeting the Wnt/β-catenin pathway in osteocytes because of its central role in bone mass regulation and bone formation in response to mechanical loading may prove useful for designing new paradigms and pharmaceuticals to treat bone disease in the future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a Target for the Ubiquitin-Proteasome Pathway. Embo J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed Y, Hayashi S, Levine A, Wieschaus E. Regulation of Armadillo by a Drosophila APC Inhibits Neuronal Apoptosis during Retinal Development. Cell. 1998;93:1171–1182. doi: 10.1016/s0092-8674(00)81461-0. [DOI] [PubMed] [Google Scholar]
- 3.Ai M, Holmen S, Van Hul W, Williams BO, Warman ML. Reduced Affinity to and Inhibition by Dkk1 Form a Common Mechanism by Which High bone Mass-Associated Missense Mutations in LRP5 Affect Canonical Wnt Signaling. Mole Cell Biol. 2005;25:4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida M, Han L, Lowe V, Warren A, Kousteni S, O'Brien CA, Manolagas S. Reactive Oxygen Species Antagonize the Skeletal Effects of Wnt/β-Catenin In Vitro and Aging Mice by Diverting β-Catenin from TCF- to FOXO-Mediated Transcription. J Bone Min Res. 2006;21 Suppl 1:S26. (abst 1092) [Google Scholar]
- 5.Anderson EJ, Kaliyamoorthy S, Iwan J, Alexander D, Knothe Tate ML. Nano-microscale models of periosteocytic flow show differences in stresses imparted to cell body and processes. Ann Biomed Eng. 2005;33:52–62. doi: 10.1007/s10439-005-8962-y. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE. Estrogen Receptor α is Required for Strain-Related β-Catenin Signaling in Osteoblasts. J Bone Min Res. 2007;22 suppl 1:S95. (abstr S064) [Google Scholar]
- 7.Asuni AA, Hooper C, Reynolds CH, Lovestone S, Anderton BH, Killick R. GSK3alpha Exhibits beta-Catenin and Tau Directed Kinase Activities that are Modulated by Wnt. Eur J Neurosci. 2006;24:3387–3392. doi: 10.1111/j.1460-9568.2006.05243.x. [DOI] [PubMed] [Google Scholar]
- 8.Babij P, Zhao W, Small C, Kharode Y, Yaworsky P, Bouxsein M, Reddy P, Bodine P, Robinson J, Bhat B, Marzolf J, Moran R, Bex F. High Bone Mass in Mice Expressing a Mutant LRP5 Gene. J Bone Miner Res. 2003;18:960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 9.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 10.Balemans W, Patel N, Ebling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willens PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Identification of a 52 kb deletion downstream of the SOST gene in pateints with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The Chromatin Remodelling Factor Brg-1 Interacts with β-Catenin to Promote Target Gene Activation. Embo J. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baylink D, Sipe J, Wergedal J, Whittemore OJ. Vitamin D-enhanced osteocytic and osteoclastic bone resorption. Am J Physiol. 1973;224:1345–1357. doi: 10.1152/ajplegacy.1973.224.6.1345. [DOI] [PubMed] [Google Scholar]
- 13.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional Interaction of an Axin Homolog, Conductin, with β-Catenin, APC and GSK3β. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 14.Bhat BM, Allen KM, Graham J, Liu W, Morales A, Anisowicz A, Lam H, McCauley C, Coleburn V, Cain M, Fortier W, Wang H, Bex FJ, Yaworsky P. Functional Modulation of LRP5-Wnt-Dkk1 Activity by Various Mutations in LRP5 Beta-Propeller 1. J Bone Miner Res. 2003;18:S46. [Google Scholar]
- 15.Bilic J, Huang Y-L, Davidson G, Zimmermann T, Cruciat C-M, Bienz M, Niehrs C. Wnt Induces LRP6 Signalsomes and Promotes Dishevelled-Dependent LRP6 Phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 16.Bodine PVN, Komm BS. Wnt Signaling and Osteoblastogenesis. Reviews in Endocrine & Metabolic Disorders. 2006;7:33–39. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 17.Bonewald L. Osteocytes. In: Marcus DFR, Nelson D, Rosen C, editors. Osteoporosis. 3rd Edition. Elsevier; 2007. [Google Scholar]
- 18.Bonewald LF. Mechanosensation and Transduction in Osteocytes. Bonekey Osteovision. 2006;3:7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High Bone Density Due to a Mutation in LDL-Receptor-Related Protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 20.Brott BK, Sokol SY. Regulation of Wnt/LRP Signaling by Distinct Domains of Dickkopf Proteins. Mol Cell Biol. 2002;22:6100–6110. doi: 10.1128/MCB.22.17.6100-6110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30:781–786. doi: 10.1016/s8756-3282(02)00707-x. [DOI] [PubMed] [Google Scholar]
- 22.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 Promotes Colon Cancer Cell Growth Through a Gs-Axin-β-Catenin Signaling Axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 23.Chen AE, Ginty DB, Fan C-M. Protein Kinase A Signalling via CREB Controls Myogenesis Induced by Wnt Proteins. Nature. 2005;433:317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- 24.Cheng B, Kato Y, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Prostaglandin E2 is essential for gap junction-mediated intercellular communication between osteocytes in response to mechanical strain. Endocrinology. 2001;142:3464–3473. doi: 10.1210/endo.142.8.8338. [DOI] [PubMed] [Google Scholar]
- 25.Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of functional gap junctions and regulation by fluid flow in osteocytelike MLO-Y4 cells. J Bone Miner Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Cherian PP, Wang X, Gu S, Bonewald LF, Sprague E, Jiang JX. Mechanical Strain Opens Connexin 43 Hemichannels in Osteocytes: A Novel Mechanism for the Release of Prostaglandin Molec Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Currey JD. The many adaptations of bone. J Biomech. 2003;36:1487–1495. doi: 10.1016/s0021-9290(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 28.DasGupta R, Fuchs E. Multiple Roles for Activated LEF/TCF Transcription Complexes during Hair Follicle Development and Differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 29.Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein Kinase 1gamma couples Wnt Receptor Activation to Cytoplasmic Signal Transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 30.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH Kinase-dependent Regulation of Glycogen synthase Kinase 3 and Protein Kinase B/AKT by the Integrin-linked Kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrlich EJ, Lanyon LE. Mechanical Strain and Bone Cell Function: A Review. Osteoporos Int. 2002;18:688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- 32.Ellies DL, Viviano B, McCarthy J, Rey J-P, Itasaki N, Saunders S, Krumlauf R. Bone Density Ligand, Sclerostin, Directly Interacts with LRP5 but not LRP5G171V to Modulate Wnt Activity. J Bone Min Res. 2006;21:1738–1749. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- 33.Essers MAG, de Vries-Smits LMM, Barker N, Polderman PE, Burgering BMT, Korswagen HC. Functional Interactional Between β-Catenin and FOXO in Oxidative Stress Signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 34.Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of Axin Involved in Protein-Protein Interactions, Wnt Pathway Inhibiiton, and Intracellular Localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farr GH, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Interaction among GSK-3, GBP, Axin, and APC in Xenopus Axis Specification. J Cell Biol. 2000;148:691–701. doi: 10.1083/jcb.148.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frost HM. Bone "Mass" and the "Mechanostat": A Proposal. Anat Record. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 37.Fukumoto S, Hsieh C-M, Maemura K, Layne MD, Yet S-F, Lee K-H, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee M-E. Akt Participation in the Wnt Signaling Pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- 38.Gandarillas A, Scholl FG, Benito N, Gamallo C, Quintanilla M. Induction of PA2.26, a cell-surface antigen expressed by active fibroblasts, in mouse epidermal keratinocytes during carcinogenesis. Mol Carcinog. 1997;20:10–18. doi: 10.1002/(sici)1098-2744(199709)20:1<10::aid-mc3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Giladi M, Milgrom C, Simkin A, Danon Y. Stress fractures. Identifialble risk factors. Am J Sports Med. 1991;19:647–653. doi: 10.1177/036354659101900617. [DOI] [PubMed] [Google Scholar]
- 40.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arsian-Kirchner M, Batch JA, Beighton P, Black GCM, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepa A, Floege B, Halfide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superta-Furga A, Swoboda W, van den Boogaard M-J, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL Receptor-Related Protein 5 (LRP5) Affects Bone Accrual and Eye Development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 41.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 42.Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA. Analysis of the Vestigial Tail Mutation Demonstrates that Wnt-3a Gene Dosage Regulates Mouse Axial Development. Genes Develop. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- 43.Gu G, Nars M, Hentunen TA, Metsikko K, Vaananen HK. Isolated primary osteocytes express functional gap junctions in vitro. Cell Tissue Res. 2006;323:263–271. doi: 10.1007/s00441-005-0066-3. [DOI] [PubMed] [Google Scholar]
- 44.Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A. 2004;101:16689–16694. doi: 10.1073/pnas.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris SE, Yang W, Harris MA, Gluhak-Heinrich J, Bonewald LF, Rowe DW, Kalajzic I. Osteocyte Gene Expression Signatures Indicate that Neural, Muscle, and Cytoskeletal Genes as well as Wnt Signaling Represent Novel Pathways for Osteocyte Function. J Bone Miner Res. 2006;21:S3. [Google Scholar]
- 46.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of β-Catenin by HUman Axin and Its Association with the APC Tumor Suppressor, β-Catenin. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann C. A Wnt Canon Orchestrating Osteoblastogenesis. Trends in Cell Biology. 2006;16:151–158. doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Heino TJ, Hentunen TA, Vaananen HK. Conditioned Medium from Osteocytes Stimulates the Proliferation of Bone Marrow Mesenchymal Stem Cells and Their Differentiation into Osteoblasts. Exp Cell Res. 2004;294:458–468. doi: 10.1016/j.yexcr.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Hillsley MV, Frangos JA. Alkaline phosphatase in osteoblasts is down-regulated by pulsatile fluid flow. Calcif Tissue Int. 1997;60:48–53. doi: 10.1007/s002239900185. [DOI] [PubMed] [Google Scholar]
- 50.Hughes DE, Salter DM, Simpson R. CD44 expression in human bone: a novel marker of osteocytic differentiation. J Bone Miner Res. 1994;9:39–44. doi: 10.1002/jbmr.5650090106. [DOI] [PubMed] [Google Scholar]
- 51.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a Negative Regulator of the Wnt Signaling Pathway, Forms a Complex with GSK-3β and β-Catenin and Promotes GSK-3β-Dependent Phosphorylation of β-Catenin. Embo J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a Context-Dependent Activator and Inhibitor of Wnt Signaling. Development. 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- 53.Itoh K, Krupnick VE, Sokol SY. Axis Determination in Xenopus Involves Biochemical Interactions of Axin, Glycogen Synthase Kinase 3 and β-Catenin. Curr Biol. 1998;8:591–598. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- 54.Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson ML, Picconi JL, Recker RR. The Gene for High Bone Mass. The Endocrinologist. 2002;12:445–453. [Google Scholar]
- 56.Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW. Dentin Matrix protein 1 Expression during Osteoblastic Differentiation, Generation of an Osteocyte GFP-Transgene. Bone. 2004;35:74–82. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Kamel MA, Holladay BR, Johnson ML. Potential Interaction of Prostaglandin and Wnt Signaling pathways Mediating Bone Cell Responses to Fluid Flow. J Bone Min Res. 2006;21 suppl 1:S92. (abs F166) [Google Scholar]
- 58.Kamel MA, Kitase Y, Kim-Weroha NA, Holladay BR, Bonewald LF, Johnson ML. Fluid Flow Shear Stress and Prostagladin E2 Activates β-Catenin Signaling in MLO-Y4 Osteocytic and 2T3 Osteoblastic Cells. J Bone Min Res. 2007;22 suppl 1:S375. (abstr W053) [Google Scholar]
- 59.Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28:145–149. doi: 10.1016/s8756-3282(00)00421-x. [DOI] [PubMed] [Google Scholar]
- 60.Kamioka H, Sugawara Y, Murshid SA, Ishihara Y, Honjo T, Takano-Yamamoto T. Fluid shear stress induces less calcium response in a single primary osteocyte than in a single osteoblast: implication of different focal adhesion formation. J Bone Miner Res. 2006;21:1012–1021. doi: 10.1359/jbmr.060408. [DOI] [PubMed] [Google Scholar]
- 61.Kato Y, Windle J, Koop B, Qiao M, Bonewald LF. Establishment of an Osteocyte-like Cell Line, MLO-Y4. J Bone Miner Res. 1997;12:2014–2023. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 62.Kitase Y, Jiang JX, Bonewald L. The Anti-apoptotic Effects of Mechanical Strain on Osteocytes are mediated by PGE2 and Monocyte Chemotactic Protein, (MCP-3); Selective protection my MCP3 against Glucocorticoid (GC) and not TNF-a induced Apoptosis. J Bone Min Res. 2006;21 suppl 1:S48. (#1177) [Google Scholar]
- 63.Kitase Y, Johnson ML, Bonewald LF. The Protective Effects of Mechanical Strain on Osteocyte Viability is Mediated by the Effects of Prostaglandin on the cAMP/PKA and β-Catenin Pathways. J Bone Min Res. 2007;22 suppl 1:S178. (abstr M237) [Google Scholar]
- 64.Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9:441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 65.Knothe Tate ML. Mixing mechanisms and net solute transport in bone. Ann Biomed Eng. 2001;29:810–811. doi: 10.1114/1.1397788. author reply 812-6. [DOI] [PubMed] [Google Scholar]
- 66.Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–117. doi: 10.1016/s8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]
- 67.Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca+2 Pathway: A New Vertebrate Wnt Signaling Pathway Takes Shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 68.Kurata K, Heino TJ, Higaki H, Vaananen HK. Bone marrow cell differentiation induced by mechanically damaged osteocytes in 3D gel-embedded culture. J Bone Miner Res. 2006;21:616–625. doi: 10.1359/jbmr.060106. [DOI] [PubMed] [Google Scholar]
- 69.Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, Kinney JH, Bonewald LF. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466–476. doi: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lanyon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int. 1993;53:S102–S106. doi: 10.1007/BF01673415. discussion S106-7. [DOI] [PubMed] [Google Scholar]
- 71.Lau K-HW, Kapur S, Kesavan C, Baylink DJ. Up-Regulation of the Wnt, Estrogen Receptor, Insulin-like Growth Factor-I, and Bone Morphogenetic Protein Pathways in C57BL/6J Osteoblasts as Opposed to C3H/HeJ Osteoblasts in Part Contributes to the Differential Anabolic Response to Fluid Shear. J Biol Chem. 2006;281:9576–9588. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- 72.Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, Harris SE, Rowe DW, Ke HZ, W D. Dkk2 has a Role in Terminal Osteoblast Differentiation and Mineralized Matrix Formation. Nature Genetics. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 73.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and Antagonizes Canonical Wnt Signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 74.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A Mutation in the LDL Receptor-Related Protein 5 Gene Results in the Autosomal Dominant High-Bone-Mass Trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma M, Sen B, Case N, Xie Z, Jo H, Gross T, Rubin J. Mechanical Activation of β-catenin is Enhanced After Caveolin-1 Knockdown. J Bone Min Res. 2007;22 suppl 1:S358. (abstr T505) [Google Scholar]
- 76.Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 78.Mao B, Wu W, Davidson G, Marhold J, Li MM, Mechler B, Delius J, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen Proteins are Dickkopf Receptors that Regulate Wnt/β-Catenin Signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 79.McGarry JG, Klein-Nulend J, Prendergast PJ. The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun. 2005;330:341–348. doi: 10.1016/j.bbrc.2005.02.175. [DOI] [PubMed] [Google Scholar]
- 80.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that Phosphorylation of GSK3 Plays in Insulin and Wnt Signaling Defined by Knockin Analysis. Embo J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mikuni-Takagaki Y, Kakai Y, Satoyoshi M, Kawano E, Suzuki Y, Kawase T, Saito S. Matrix mineralization and the differentiation of osteocyte-like cells in culture. J Bone Miner Res. 1995;10:231–242. doi: 10.1002/jbmr.5650100209. [DOI] [PubMed] [Google Scholar]
- 82.Mikuni-Takagaki Y, Suzuki Y, Kawase T, Saito H. Distinct Responses of Different Populations of Bone Cells to Mechanical Stress. Endocrinology. 1996;137:2028–2035. doi: 10.1210/endo.137.5.8612544. [DOI] [PubMed] [Google Scholar]
- 83.Mlodzik M. Planar Cell Polarization: Do the Same Mechanisms Regulate Drosophila tissue Polarity and Vertebrate Gastrulation? Trends Genet. 2002;18:564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 84.Morvan F, Boulukos K, Clement-Lacroix P, Roman-Roman S, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G. Deletion of a Single Allele of the Dkk1 Gene Leads to an Increase in Bone Formation and Bone Mass. J Bone Min Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 85.Nakamura T, Hamada F, Ihidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Axin, an Inhibitor of the Wnt Signalling Pathway, Interacts with β-Catenin, GSK-3 βand APC and Reduces the β-Catenin Level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- 86.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 87.Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39:1735–1743. doi: 10.1016/j.jbiomech.2005.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nicolella DP, Nicholls AE, Lankford J, Davy DT. Machine vision photogrammetry: a technique for measurement of microstructural strain in cortical bone. J Biomech. 2001;34:135–139. doi: 10.1016/s0021-9290(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 89.Nijweide PJ, Mulder RJ. Identification of osteocytes in osteoblast-like cell cultures using a monoclonal antibody specifically directed against osteocytes. Histochemistry. 1986;84:342–347. doi: 10.1007/BF00482961. [DOI] [PubMed] [Google Scholar]
- 90.Nijweide PJ, van der Plas A, Scherft JP. Biochemical and histological studies on various bone cell preparations. Calcif Tissue Int. 1981;33:529–540. doi: 10.1007/BF02409485. [DOI] [PubMed] [Google Scholar]
- 91.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid Shear Stress Induces β-Catenin Signaling in Osteoblasts. Calcif Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 92.Ominsky MS, Stouch B, Doellgas G, Gong J, Cao J, Tipton B, Haldankar R, Winters A, Chen Q, Graham K, Zhou L, Hale M, Henry A, Lightwood D, Moore A, Popplewell A, Robinson M, Vlasseros F, Jolette J, Smith SY, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Administration of Sclerostin Monoclonal Antibodies to Female Cynomolgus Monkeys Results in Increased Bone Formation, Bone Mineral Density and Bone Strength. J Bone Min Res. 2006;21 suppl 1:S44. (abs 1162) [Google Scholar]
- 93.Ominsky MS, Warmington KS, Asuncion FJ, Tan HL, Grisanti MS, Geng Z, Stephens P, Henry A, Lawson A, Lightwood D, Perkins V, Kirby H, Moore A, Robinson M, Li X, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Sclerostin Monoclonal Antibody Treatment Increases Bone Strength in Aged Osteopenic Ovariectomized Rats. J Bone Min Res. 2006;21 suppl 1:S44. (abs 1161) [Google Scholar]
- 94.Oster GF, Murray JD, Harris AK. Mechanical aspects of mesenchymal morphogenesis. J Embryol Exp Morphol. 1983;78:83–125. [PubMed] [Google Scholar]
- 95.Peters JM, McKay RM, McKay JP, Graff JM. Casein Kinase I Transduces Wnt Signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 96.Piconni JL, Johnson ML. Biphasic Expression of Cox-2 in REsponse to Pulsatile Fluid Flow. J Bone Miner Res. 2004;19:S392. [Google Scholar]
- 97.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-Receptor-Related Protein Mediates Wnt Signalling in Mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 98.Ponik SM, Triplett JW, Pavalko FM. Osteoblasts and Osteocytes Respond Differently to Oscillatory and Unidirectional Fluid Flow Profiles. J Cell Biochem. 2007;100:794–807. doi: 10.1002/jcb.21089. [DOI] [PubMed] [Google Scholar]
- 99.Quaiser T, Anton R, Kuhl M. Kinases and G Proteins Join the Wnt Receptor Complex. BioEssays. 2006;28:339–343. doi: 10.1002/bies.20386. [DOI] [PubMed] [Google Scholar]
- 100.Rath Bonivtch A, Bonewald LF, Nicolella DP. Tissue strain amplification at the osteocyte lacuna: A microstructural finite element analysis. J Biomech. 2006 doi: 10.1016/j.jbiomech.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rawlinson SC, Pitsillides AA, Lanyon LE. Involvement of Different Ion Channels in Osteoblasts' and Osteocytes' Early Responses to Mechanical Strain. Bone. 1996;19:609–614. doi: 10.1016/s8756-3282(96)00260-8. [DOI] [PubMed] [Google Scholar]
- 102.Rawlinson SCF, El Haj AJ, Minster SL, Tavares IA, Bennett A, Lanyon LE. Loading-related Increases in Prostaglandin Production in Cores of Adult Canine Cancellous Bone In Vitro: a Role for Prostacyclin in Adaptive Bone Remodeling? J Bone Miner Res. 1991;6:1345–1357. doi: 10.1002/jbmr.5650061212. [DOI] [PubMed] [Google Scholar]
- 103.Reich KM, Gay CV, Frangos JA. Fluid Shear Stress as a Mediator of Osteoblast Cyclic Adenosine Monophosphate Prduction. J Cell Physiol. 1990;143:100–104. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- 104.Robinson JA, Chatterjee-Kishore M, Yaworsky P, Cullen DM, Zhao W, Li C, Kharode YP, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/β-Catenin Signaling is a Normal Physiological Response to Mechanical Loading in Bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 105.Robling AG, Bellido TM, Turner CH. Mechanical Loading Reduces Osteocyte Expression of Sclerostin Protein. J Bone Min Res. 2006;21 suppl 1:S72. (abs 1275) [PubMed] [Google Scholar]
- 106.Rubin CT, Lanyon LE. Regulation of Bone Formation by Applied Dynamic Loads. J Bone Joint Surgery Am. 1984;66A:397–402. [PubMed] [Google Scholar]
- 107.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 108.Sakanaka C, Weiss JB, Williams LT. Bridging of β-Catenin and Glycogen Syhase Kinase-3b by Axin and Inhibiiton of β-Catenin-mediated Transcription. Proc Natl Acad Sci USA. 1998;95:3020–3030. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The Wnt Co-Receptor Lrp5 is Essential for Skeletal Mechanotransduction, but Not for the Anabolic Bone Response to Parathyroid Hormone Treatment. J Biol Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 110.Steck R, Niederer P, Knothe Tate ML. A finite element analysis for the prediction of load-induced fluid flow and mechanochemical transduction in bone. J Theor Biol. 2003;220:249–259. doi: 10.1006/jtbi.2003.3163. [DOI] [PubMed] [Google Scholar]
- 111.Sun N, Gao Y, Pretoriu J, Morony S, Kostenuik PJ, Simonet S, Lacey DL, Sarosi I, Kurahara C, Patszty C. High bone mineral density in SOST knockout mice demonstractes functional conservation of osteocyte mediated bone homeostasis in mouse and human. J.Bone.Min. Res. 2003;18 Suppl. 2:S7. [Google Scholar]
- 112.Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, Williams LT. PAR-1 is a Dishevelled-associated Kinase and a Positive Regulator of Wnt Signaling. Nat Cell Biol. 2001;3:628–636. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- 113.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 114.Tazawa K, Hoshi K, Kawamoto S, Tanaka M, Ejiri S, Ozawa H. Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. J Bone Miner Metab. 2004;22:524–529. doi: 10.1007/s00774-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 115.van Bezooijen RL, Svensson JP, Eefting D, Visser A, van der Horst G, Karperien M, Quax PH, Vrieling H, Papapoulos SE, ten Dijke P, Lowik CW. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res. 2007;22:19–28. doi: 10.1359/jbmr.061002. [DOI] [PubMed] [Google Scholar]
- 116.van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an Osteocyte-Derived Negative Modulator of Bone Formation. Cytokine Growth Factor Res. 2005;16:319–327. doi: 10.1016/j.cytogfr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 117.Vatsa A, Mizuno D, Smit TH, Schmidt CF, MacKintosh FC, Klein-Nulend J. Bio imaging of intracellular NO production in single bone cells after mechanical stimulation. J Bone Miner Res. 2006;21:1722–1728. doi: 10.1359/jbmr.060720. [DOI] [PubMed] [Google Scholar]
- 118.Veno P, Nicolella DP, Sivakumar P, Kalajzic I, Rowe D, Harris SE, Bonewald L, Dallas SL. Live Imaging of Osteocytes Within Their Lacunae Reveals Cell Body and Dendrite Motions. J. Bone. Min. Res. 2006 in press. [Google Scholar]
- 119.Wadhwa S, Choudhary S, Voznesensky M, Epstein MA, Raisz LG, Pilbeam CC. Fluid flow Induces COX-2 Expression in MC3T3-E1 Osteoblasts via a PKA Signaling Pathway. Biochem Biophys Res Commun. 2002;297:46–51. doi: 10.1016/s0006-291x(02)02124-1. [DOI] [PubMed] [Google Scholar]
- 120.Wadhwa S, Godwin SL, Peterson DR, Epstein MA, Raisz LG, Pilbeam CC. Fluid Flow Induction of Cyclo-oxygenase 2 Gene Expression in Osteoblasts is Dependent on an Extracellular Signal Regulated Kinase Signaling Pathway. J Bone Miner Res. 2002;17:266–274. doi: 10.1359/jbmr.2002.17.2.266. [DOI] [PubMed] [Google Scholar]
- 121.Wang L, Cowin SC, Weinbaum S, Fritton SP. Modeling tracer transport in an osteon under cyclic loading. Ann Biomed Eng. 2000;28:1200–1209. doi: 10.1114/1.1317531. [DOI] [PubMed] [Google Scholar]
- 122.Wang L, Fritton SP, Cowin SC, Weinbaum S. Fluid pressure relaxation depends upon osteonal microstructure: modeling an oscillatory bending experiment. J Biomech. 1999;32:663–672. doi: 10.1016/s0021-9290(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 123.Wang L, Fritton SP, Weinbaum S, Cowin SC. On bone adaptation due to venous stasis. J Biomech. 2003;36:1439–1451. doi: 10.1016/s0021-9290(03)00241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weinbaum S, Cowin SC, Zeng YA. Model for the Excitation of Osteocytes by Mechanical Loading-Induced Bone Fluid Shear Stresses. J. Biomechanics. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 125.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of Osteoblastogenesis and Promotion of Apoptosis of Osteoblasts and Osteocytes by Glucocorticoids. Potential Mechanisms of their Deleterious Effects on Bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Westendorf JJ, Kahler RA, Schroeder TM. Wnt Signaling in Osteoblasts and Bone Diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 127.Willert K, Brink M, Wodarz a, Varmus H, Nusse R. Casein Kinase 2 Associates with and Phosphorylates Dishevelled. Embo J. 1997;16:3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Williams JL, Iannotti JP, Ham A, Bleuit J, Chen JH. Effects of fluid shear stress on bone cells. Biorheology. 1994;31:163–170. doi: 10.3233/bir-1994-31204. [DOI] [PubMed] [Google Scholar]
- 129.Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Calvet JP, Bonewald L, Quarles LD. Polycystin-1: A novel mechanosensor in osteoblast/osteocyte coupled to Runx2. 2006 submitted. [Google Scholar]
- 130.Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A. Axil, a Member of the Axin Family, Interacts with Both Glycogen Synthase Kinase 3β and β-Catenin and Inhibits Axis Formation of Xenopus Embryos. Mol Cell Biol. 1998;18:2867–2875. doi: 10.1128/mcb.18.5.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan K-L, MacDougald OA, Brown AMC, Evan G, Kitajewski J, Wang C-Y. Wnt Signaling Promotes Oncogenic Transformation by Inhibiting c-Myc-induced Apoptosis. J Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, Bonewald LF. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–4552. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res. 2002;17:2068–2079. doi: 10.1359/jbmr.2002.17.11.2068. [DOI] [PubMed] [Google Scholar]


