Abstract
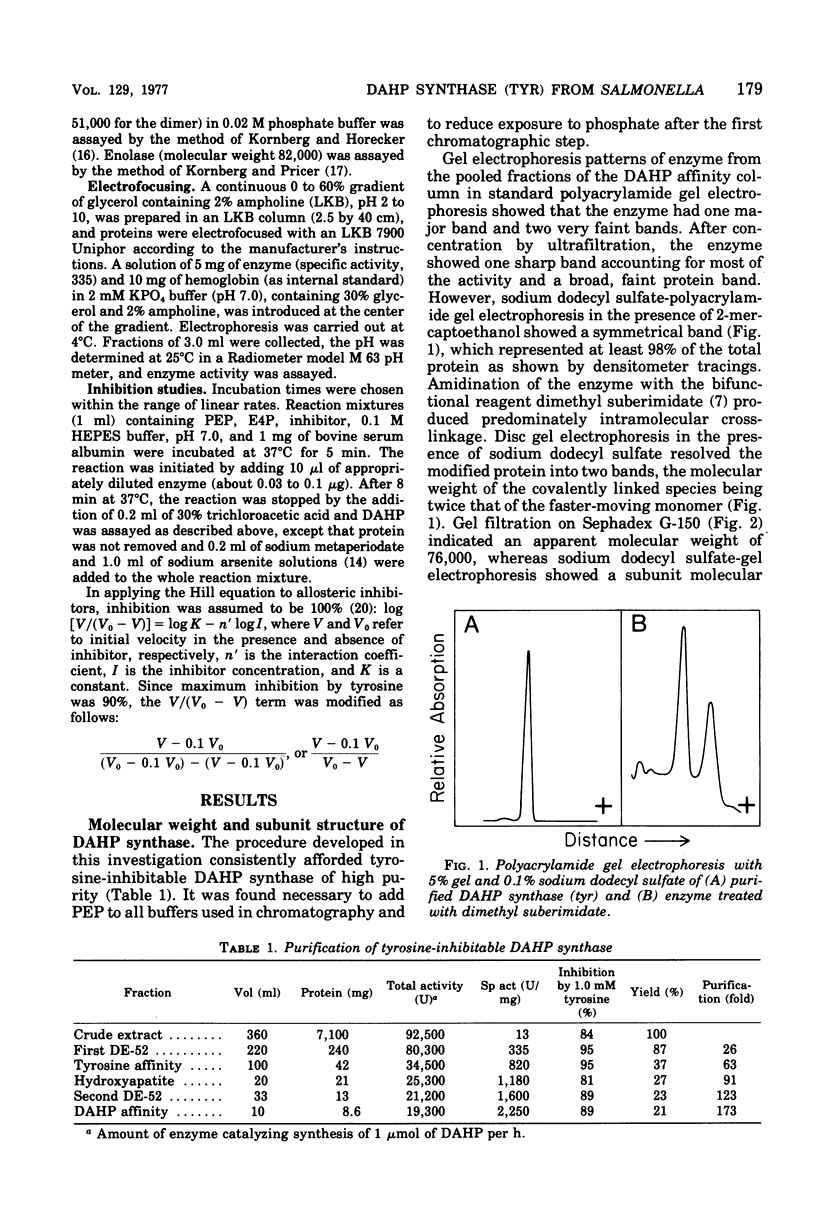
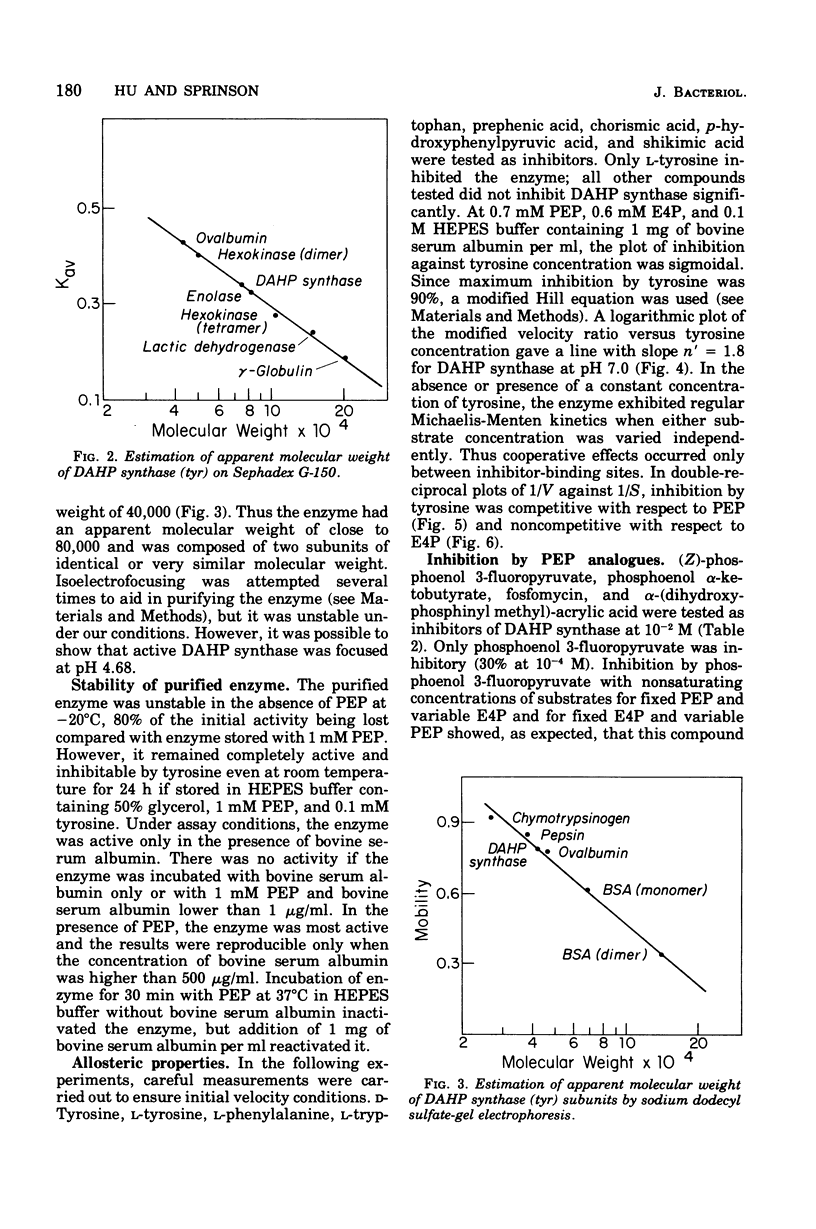
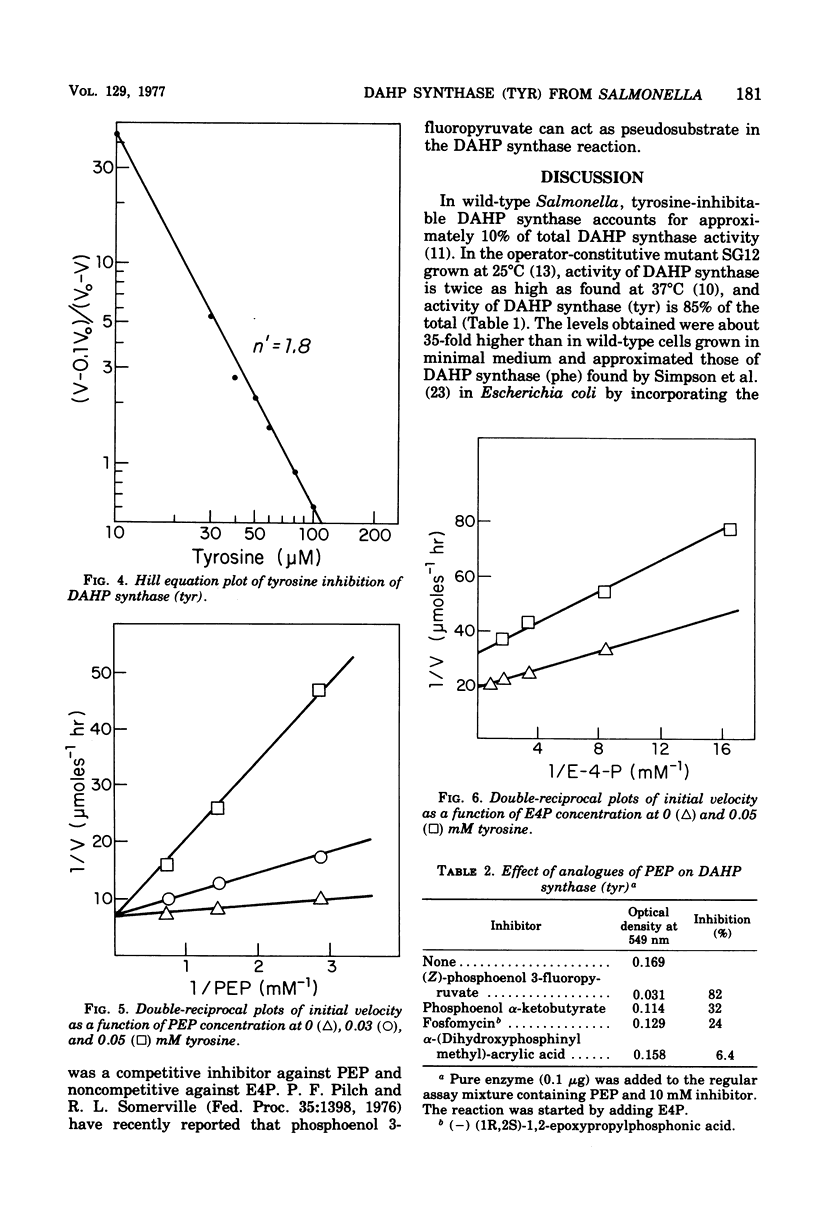
Tyrosine-inhibitable 3-deoxy-D-arabinoheptulosonic acid-7-phosphate (DAHP) synthase was purified to homogeneity without significant loss of sensitivity to inhibition by tyrosine from an operator-constitutive strain (tyrOc) of Salmonella. The enzyme had an apparent molecular weight of 76,000 by gel filtration and a subunit molecular weight of 40,000 by sodium dodecyl sulfate-gel electrophoresis and by reaction with dimethyl suberimidate. It had an isoelectric point of 4.68. Inhibition by L-tyrosine showed a Hill coefficient of 1.8 at pH 7.0, suggesting cooperative interaction between tyrosine-binding sites, and was competitive with phosphoenol pyruvate and noncompetitive with erythrose-4-phosphate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondinell W. E., Sprinson D. B. The stereochemistry of pyruvate kinase. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1464–1467. doi: 10.1016/0006-291x(70)90032-x. [DOI] [PubMed] [Google Scholar]
- Cassidy P. J., Kahan F. M. A stable enzyme-phosphoenolpyruvate intermediate in the synthesis of uridine-5'-diphospho-N-acetyl-2-amino-2-deoxyglucose 3-O-enolpyruvyl ether. Biochemistry. 1973 Mar 27;12(7):1364–1374. doi: 10.1021/bi00731a017. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo A. B., Dayan J., Sprinson D. B. Purification and kinetics of tyrosine-sensitive 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthetase from Salmonella. J Biol Chem. 1973 Apr 10;248(7):2344–2353. [PubMed] [Google Scholar]
- DeLeo A. B., Sprinson D. B. 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate synthase mutants of Salmonella typhimurium. J Bacteriol. 1975 Dec;124(3):1312–1320. doi: 10.1128/jb.124.3.1312-1320.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub E. G., Liu K. P., Sprinson D. B. tyrR, a regulatory gene of tyrosine biosynthesis in Salmonella typhimurium. J Bacteriol. 1973 Sep;115(3):1094–1102. doi: 10.1128/jb.115.3.1094-1102.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub E., Zalkin H., Sprinson D. B. Correlation of genes and enzymes, and studies on regulation of the aromatic pathway in Salmonella. J Biol Chem. 1967 Nov 25;242(22):5323–5328. [PubMed] [Google Scholar]
- Hendlin D., Stapley E. O., Jackson M., Wallick H., Miller A. K., Wolf F. J., Miller T. W., Chaiet L., Kahan F. M., Foltz E. L. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969 Oct 3;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic phosphorylation of adenosine and 2,6-diaminopurine riboside. J Biol Chem. 1951 Dec;193(2):481–495. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapidot Y., de Groot N. The chemical synthesis and the biochemical properties of peptidyl-tRNA. Prog Nucleic Acid Res Mol Biol. 1972;12:189–228. doi: 10.1016/s0079-6603(08)60663-7. [DOI] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Chang H. W., Chen Y. T. Purification of acetylcholinesterase by affinity chromatography and determination of active site stoichiometry. J Biol Chem. 1972 Mar 10;247(5):1555–1565. [PubMed] [Google Scholar]
- SPRINSON D. B., ROTHSCHILD J., SPRECHER M. THE SYNTHESIS OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE. J Biol Chem. 1963 Oct;238:3170–3175. [PubMed] [Google Scholar]
- Simpson R. J., Davidson B. E., Dopheide T. A., Andrews S., Pittard J. Purification and properties of 3-deoxy-d-arabinoheptulosonic acid-7-phosphate synthetase (phe) from a lambda aroG+ transductant of Escherichia coli. J Bacteriol. 1971 Sep;107(3):798–805. doi: 10.1128/jb.107.3.798-805.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe J. A., Kenyon G. L. Analogs of phosphoenolpyruvate. Substrate specificities of enolase and pyruvate kinase from rabbit muscle. Biochemistry. 1972 Feb 1;11(3):338–345. doi: 10.1021/bi00753a005. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


