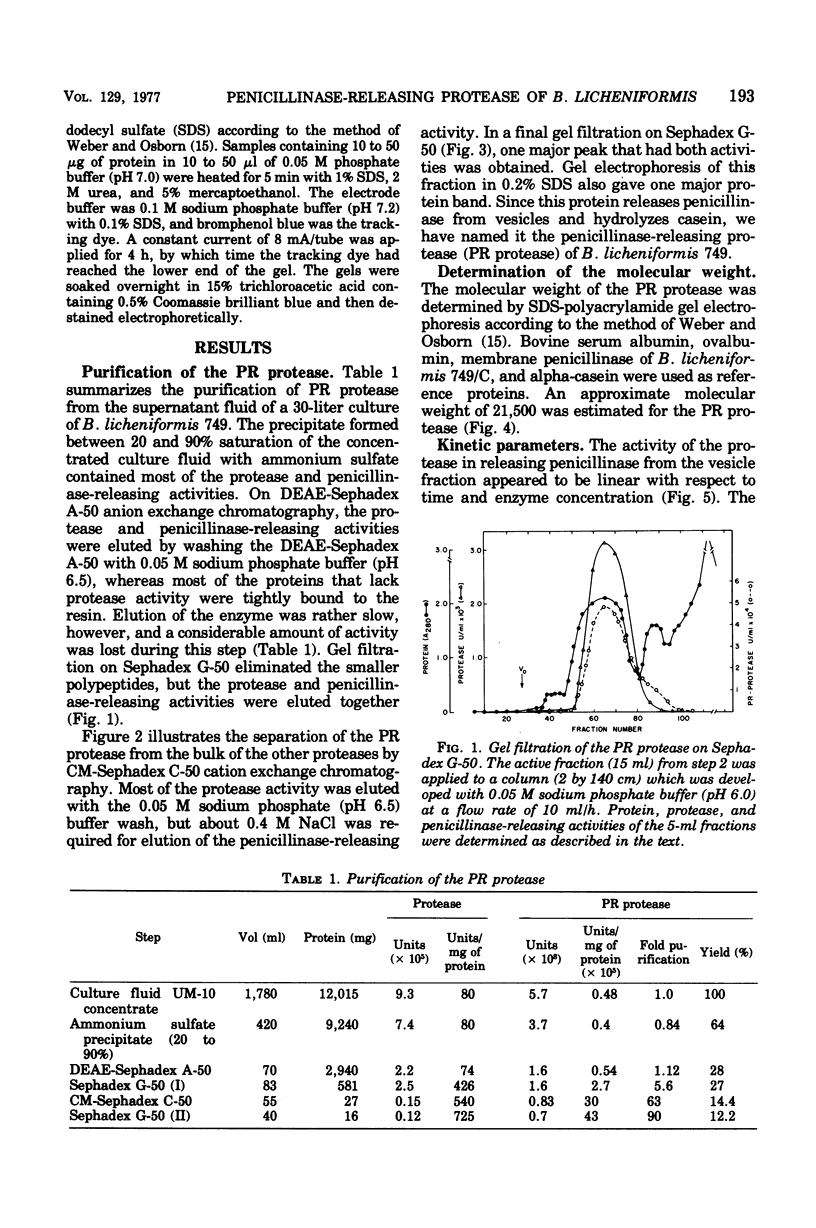
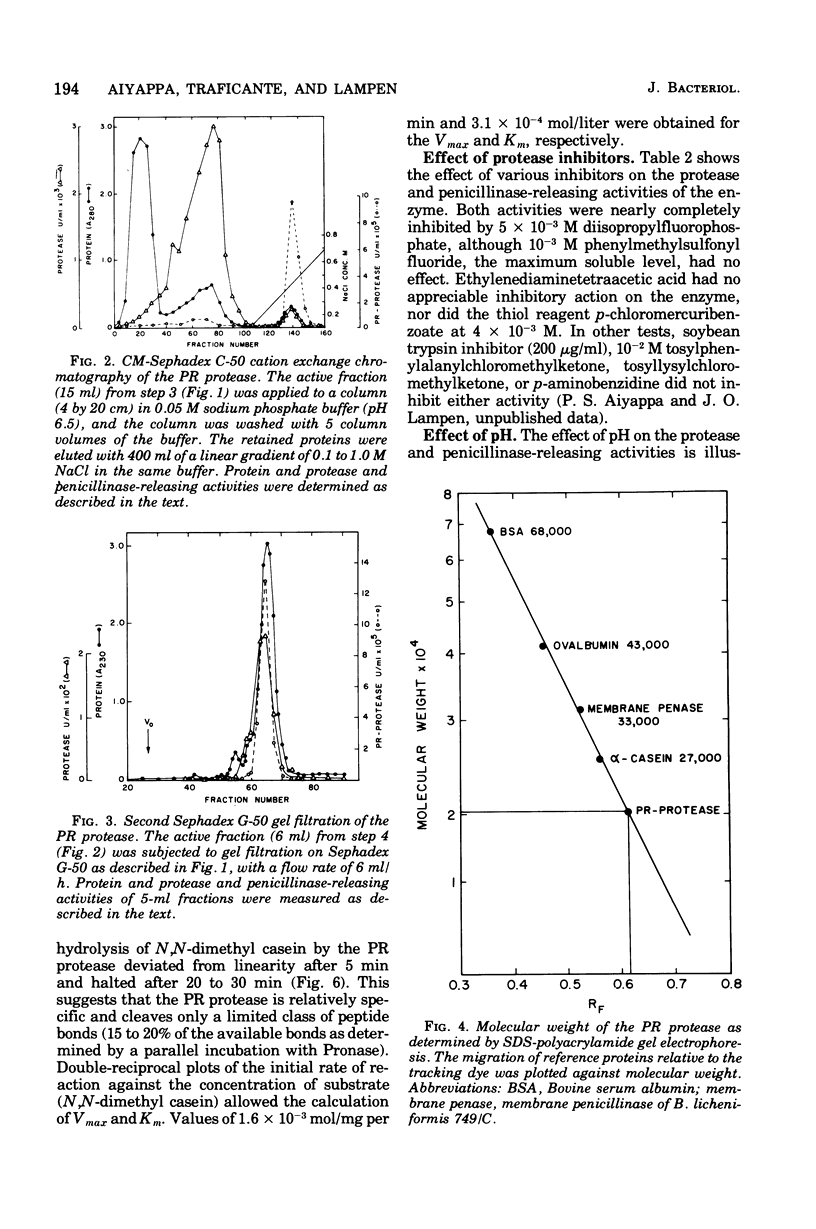
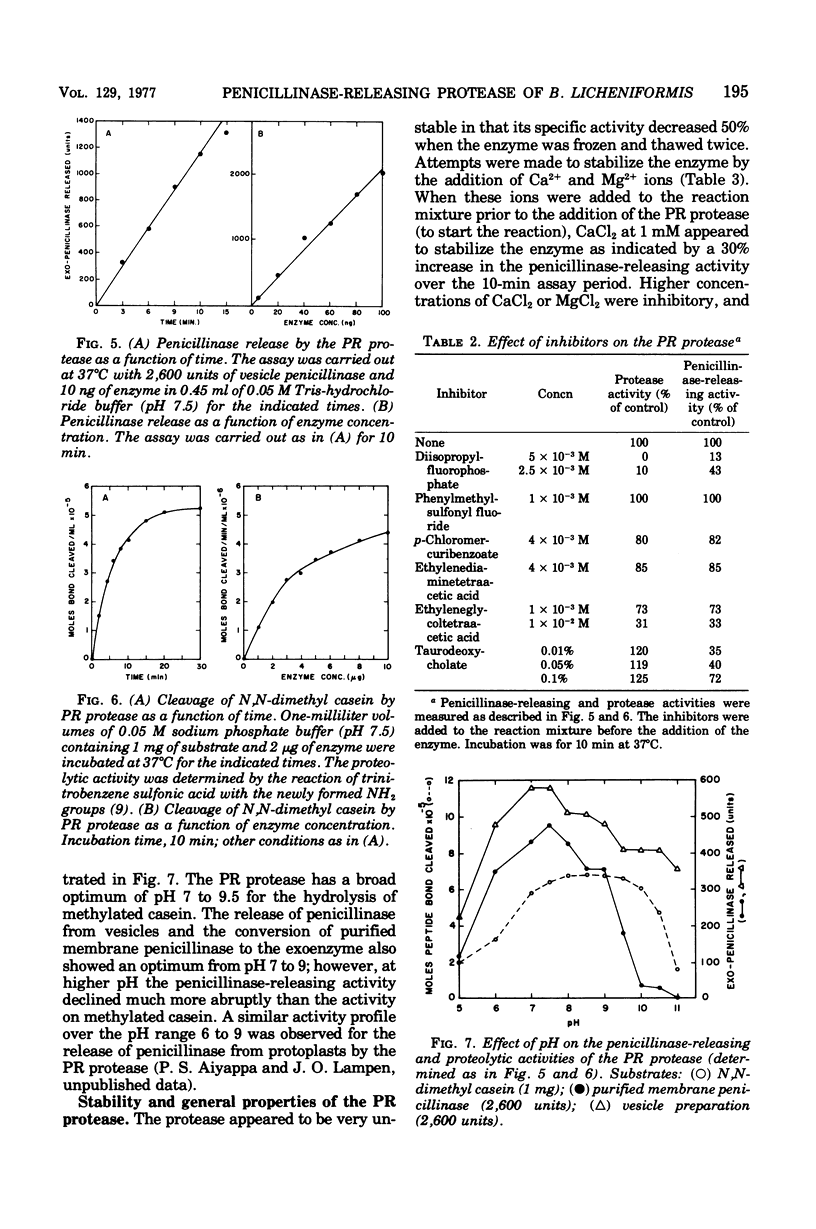
Abstract
The membrane penicillinase of Bacillus licheniformis 749/C is a phospholipoprotein which differs from the exoenzyme in that it has an additional sequence of 24 amino acid residues and a phosphatidylserine at the NH2 terminus. In exponential-phase cultures, the conversion of membrane penicillinase to exoenzyme occurs at neutral and alkaline pH. An enzyme that will cleave the membrane penicillinase to yield the exoenzyme is present (in small amounts) in exponential-phase cells and is released during their conversion to protoplasts. The enzyme is found in the filtrate of a stationary-phase culture of the uninduced penicillinase-inducible strain 749 and has been purified to apparent homogeneity from this source. The protease has an approximate molecular weight of 21,500 and requires Ca2+ ions for stabilization. It has a pH optimum of 7.0 to 9.5 for hydrolysis of casein and for the cleavage of membrane penicillinase. Both activities are inhibited by diisopropylfluorophosphate; hence, the enzyme is a serine protease. This enzyme may be entirely responsible for the formation of exopenicillinase by this organism, since the other neutral and alkaline proteases of strain 749 have little, if any, activity in releasing exopenicillinase. The enzyme has been termed penicillinase-releasing protease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Meadway R. J. Chemical structure of bacterial penicillinases. Nature. 1969 Apr 5;222(5188):24–26. doi: 10.1038/222024a0. [DOI] [PubMed] [Google Scholar]
- Crane L. J., Lampen J. O. Bacillus licheniformis 749-C plasma membrane penicillinase, a hydrophobic polar protein. Arch Biochem Biophys. 1974 Feb;160(2):655–666. doi: 10.1016/0003-9861(74)90443-3. [DOI] [PubMed] [Google Scholar]
- Dancer B. N., Lampen J. O. In vitro synthesis of hydrophobic penicillinase in extracts of Bacillus licheniforms 749/C. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1357–1364. doi: 10.1016/0006-291x(75)90509-4. [DOI] [PubMed] [Google Scholar]
- JANSEN E. F., BALLS A. K. The inhibition of beta- and gamma-chymotrypsin and trypsin by diisopropyl fluorophosphate. J Biol Chem. 1952 Feb;194(2):721–727. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O. Release of penicillinase by Bacillus licheniformis. J Gen Microbiol. 1967 Aug;48(2):261–268. doi: 10.1099/00221287-48-2-261. [DOI] [PubMed] [Google Scholar]
- Lin Y., Means G. E., Feeney R. E. The action of proteolytic enzymes on N,N-dimethyl proteins. Basis for a microassay for proteolytic enzymes. J Biol Chem. 1969 Feb 10;244(3):789–793. [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Characteristics of penicillinase release by washed cells of Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1231–1239. doi: 10.1128/jb.96.4.1231-1239.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Characteristics of penicillinase secretion by growing cells and protoplasts of Bacillus licheniformis. J Bacteriol. 1969 Feb;97(2):820–826. doi: 10.1128/jb.97.2.820-826.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Lampen J. O. Purification and characteristics of plasma membrane penicillinase from Bacillus licheniformis 749-C. J Biol Chem. 1974 Oct 10;249(19):6288–6294. [PubMed] [Google Scholar]
- Traficante L. J., Lampen J. O. Vesicle penicillinase of Bacillus licheniformis: existence of periplasmic-releasing factor(s). J Bacteriol. 1977 Jan;129(1):184–190. doi: 10.1128/jb.129.1.184-190.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. Membrane penicillinase of Bacillus licheniformis 749/C, a phospholipoprotein. J Biol Chem. 1975 Apr 25;250(8):3212–3213. [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. Membrane penicillinase of Bacillus licheniformis 749/C:sequence and possible repeated tetrapeptide structure of the phospholipopeptide region. Proc Natl Acad Sci U S A. 1976 May;73(5):1457–1461. doi: 10.1073/pnas.73.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. Purification of plasma membrane penicillinase from Bacillus licheniformis 749/C and comparison with exoenzyme. J Biol Chem. 1976 Jul 10;251(13):4095–4101. [PubMed] [Google Scholar]


