Abstract
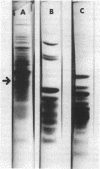
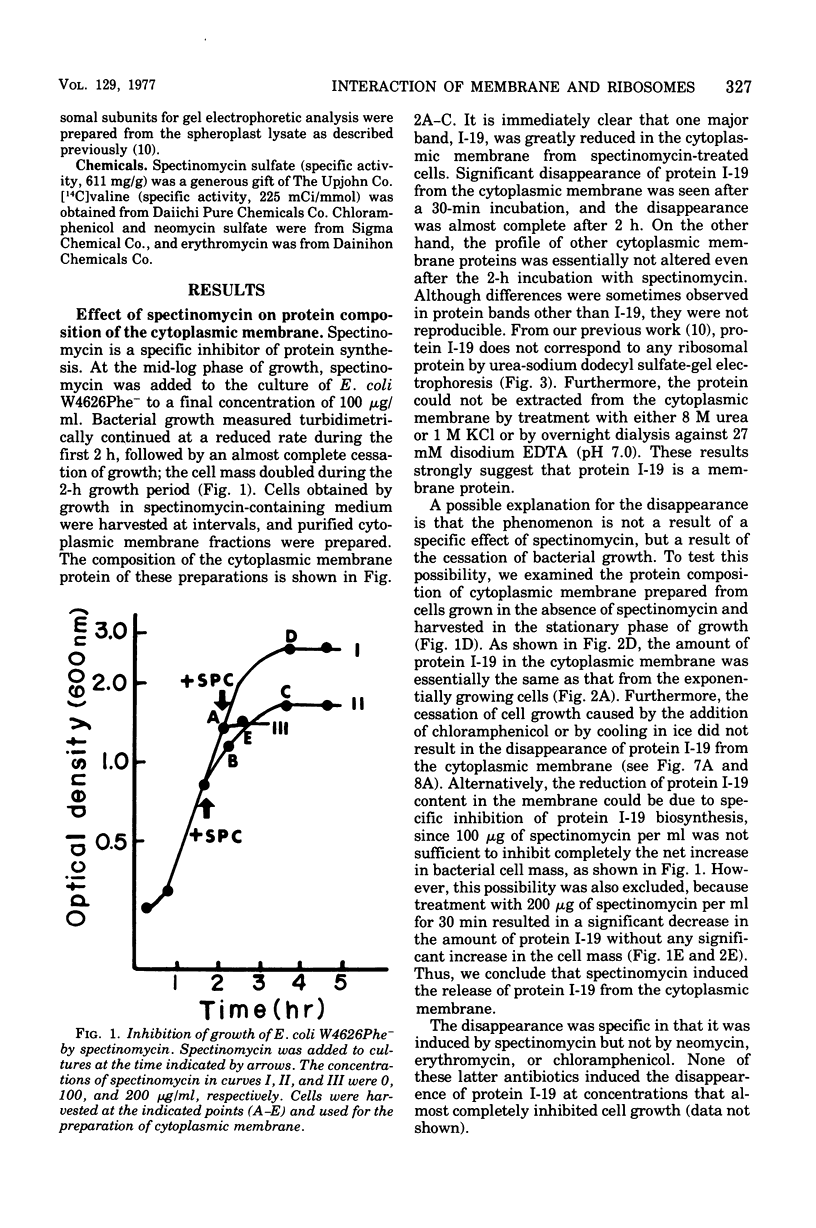
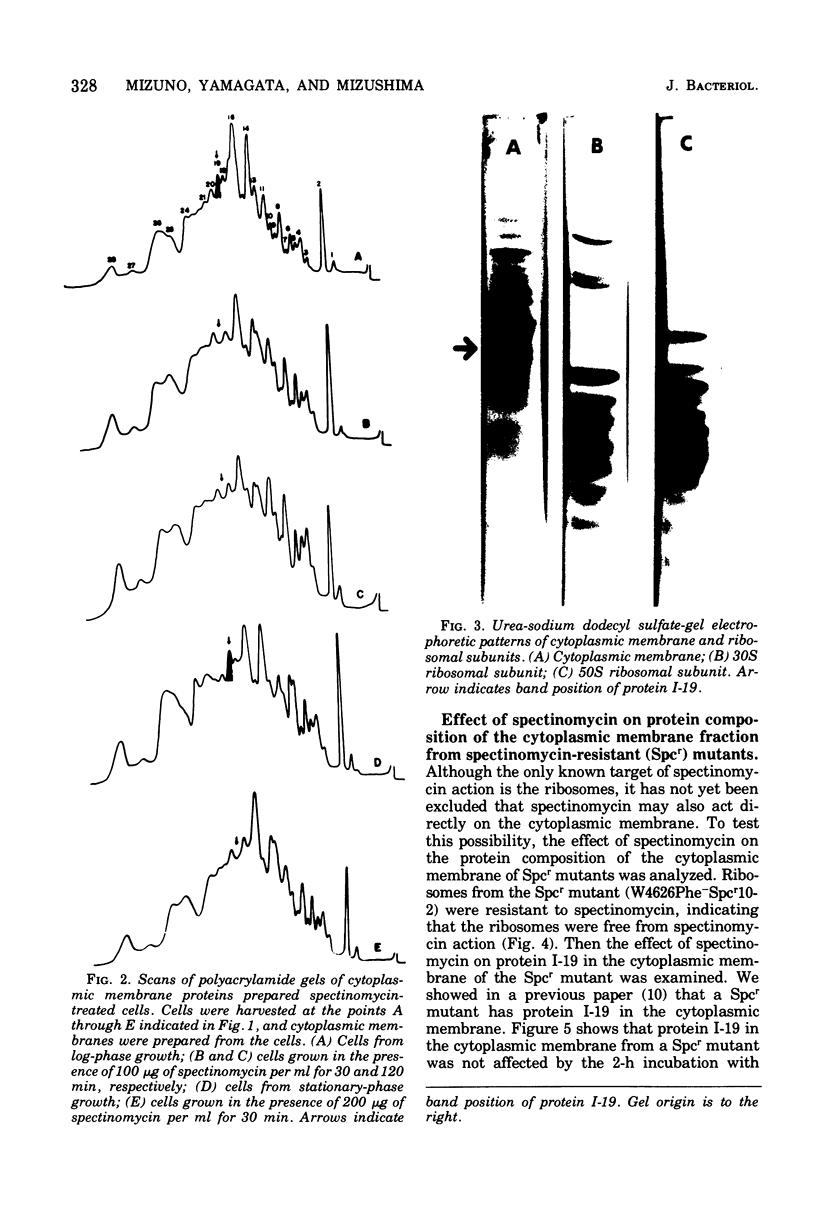
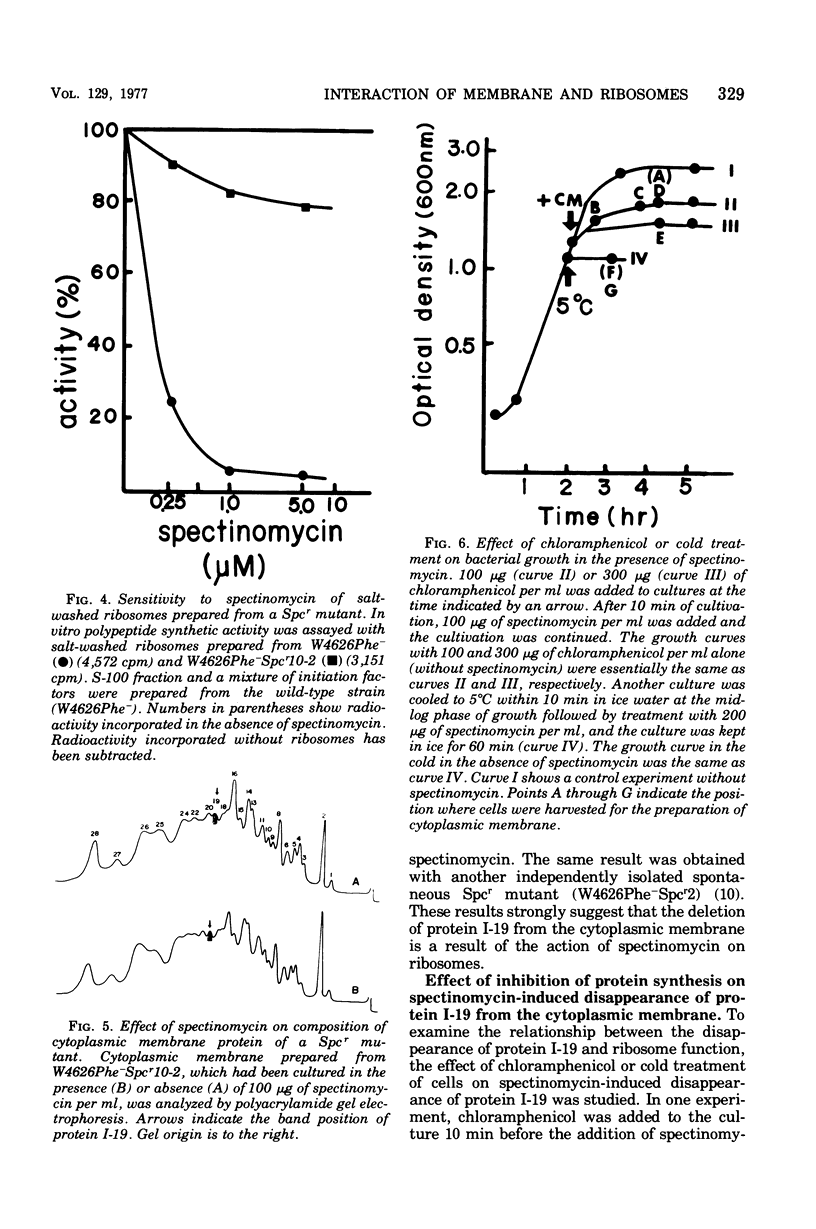
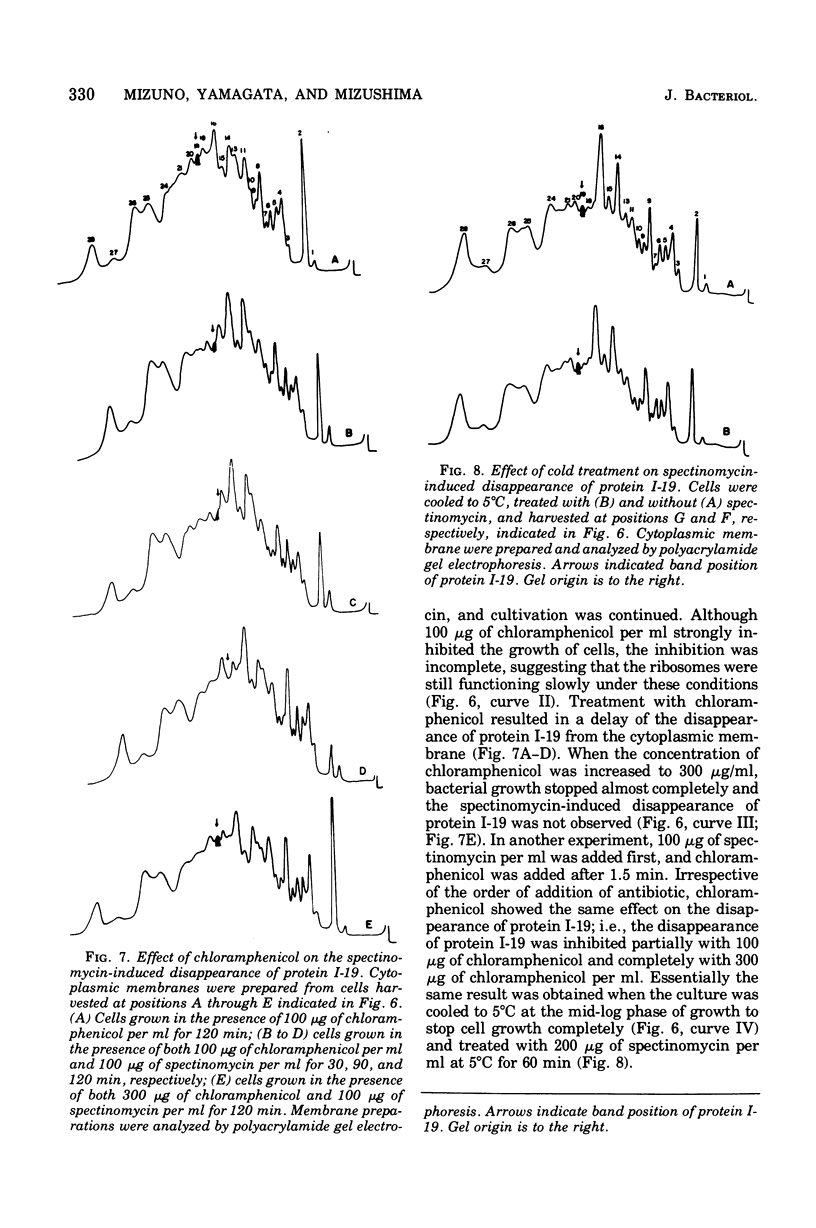
Incubation of Escherichia coli with spectinomycin caused the disappearance of a major protein from the cytoplasmic membrane. This protein, called "I-19", was not a ribosomal protein. Its disappearance was not a result of the direct action of spectinomycin on the cytoplasmic membrane, but a result of its action on ribosomes. The disappearance was specifically induced by spectinomycin, and other antibiotics such as neomycin, erythromycin, and chloramphenicol had no effect. Although growth was not required for spectinomycin-induced disappearance of protein I-19 from the cytoplasmic membrane, the disappearance was not observed under conditions where protein synthesis was inhibited completely either by the addition of chloramphenicol or by cooling in ice. It is suggested that at least some ribosomes interact with the cytoplasmic membrane and that a modification of the mode of interaction through the action of spectinomycin on ribosomes caused the deletion of membrane protein I-19.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Davies J., Davis B. D. Effect of spectinomycin on polypeptide synthesis in extracts of Escherichia coli. J Mol Biol. 1967 Oct 14;29(1):203–215. doi: 10.1016/0022-2836(67)90191-x. [DOI] [PubMed] [Google Scholar]
- Anderson P. Sensitivity and Resistance to Spectinomycin in Escherichia coli. J Bacteriol. 1969 Nov;100(2):939–947. doi: 10.1128/jb.100.2.939-947.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A., Helser T., Yamada T., Davies J. Altered ribosomes in antibiotic-resistant mutants of E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:95–100. doi: 10.1101/sqb.1969.034.01.015. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Localization of polyribosomes containing alkaline phosphatase nascent polypeptides on membranes of Escherichia coli. J Bacteriol. 1974 Jan;117(1):290–301. doi: 10.1128/jb.117.1.290-301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E. Intracellular distribution of ribosomes and polyribosomes in Bacillus megaterium. J Mol Biol. 1970 Sep 28;52(3):467–481. doi: 10.1016/0022-2836(70)90413-4. [DOI] [PubMed] [Google Scholar]
- Davies J., Anderson P., Davis B. D. Inhibition of protein synthesis by spectinomycin. Science. 1965 Sep 3;149(3688):1096–1098. doi: 10.1126/science.149.3688.1096. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Specialized transducing phages for ribosomal protein genes of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jan;72(1):6–10. doi: 10.1073/pnas.72.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y., Yamagata H. Sucrose-dependent spectinomycin-resistant mutants of Escherichia coli. J Bacteriol. 1976 Jan;125(1):142–148. doi: 10.1128/jb.125.1.142-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Mizushima S. Characterization of stuck ribosomes induced by neomycin in vivo and in vitro. Biochim Biophys Acta. 1974 Jun 14;353(1):69–76. doi: 10.1016/0005-2787(74)90098-7. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Yamada H. Isolation and characterization of two outer membrane preparations from Escherichia coli. Biochim Biophys Acta. 1975 Jan 14;375(1):44–53. doi: 10.1016/0005-2736(75)90071-1. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- SCHLESSINGER D., MARCHESTI V. T., KWAN B. C. BINDING OF RIBOSOMES TO CYTOPLASMIC RETICULUM OF BACILLUS MEGATERIUM. J Bacteriol. 1965 Aug;90:456–466. doi: 10.1128/jb.90.2.456-466.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio F. "Compartmentalization" of Escherichia coli ribosomes and ribonucleic acid. J Bacteriol. 1972 Mar;109(3):1284–1294. doi: 10.1128/jb.109.3.1284-1294.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Tai P. C., Davis B. D. Selective inhibition of initiating ribosomes by spectinomycin. Proc Natl Acad Sci U S A. 1974 May;71(5):1634–1638. doi: 10.1073/pnas.71.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Chromosomal mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jan 28;63(2):281–294. doi: 10.1016/0022-2836(72)90375-0. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Spectinomycin resistance mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jun 28;67(3):533–535. doi: 10.1016/0022-2836(72)90472-x. [DOI] [PubMed] [Google Scholar]
- van Knippenberg P. H., Duijts G. A., Euwe M. S. Polyribosomes of Escherichia coli. I. Isolation of polysomes from a complex of DNA and membrane. Mol Gen Genet. 1971;112(3):197–207. doi: 10.1007/BF00269172. [DOI] [PubMed] [Google Scholar]