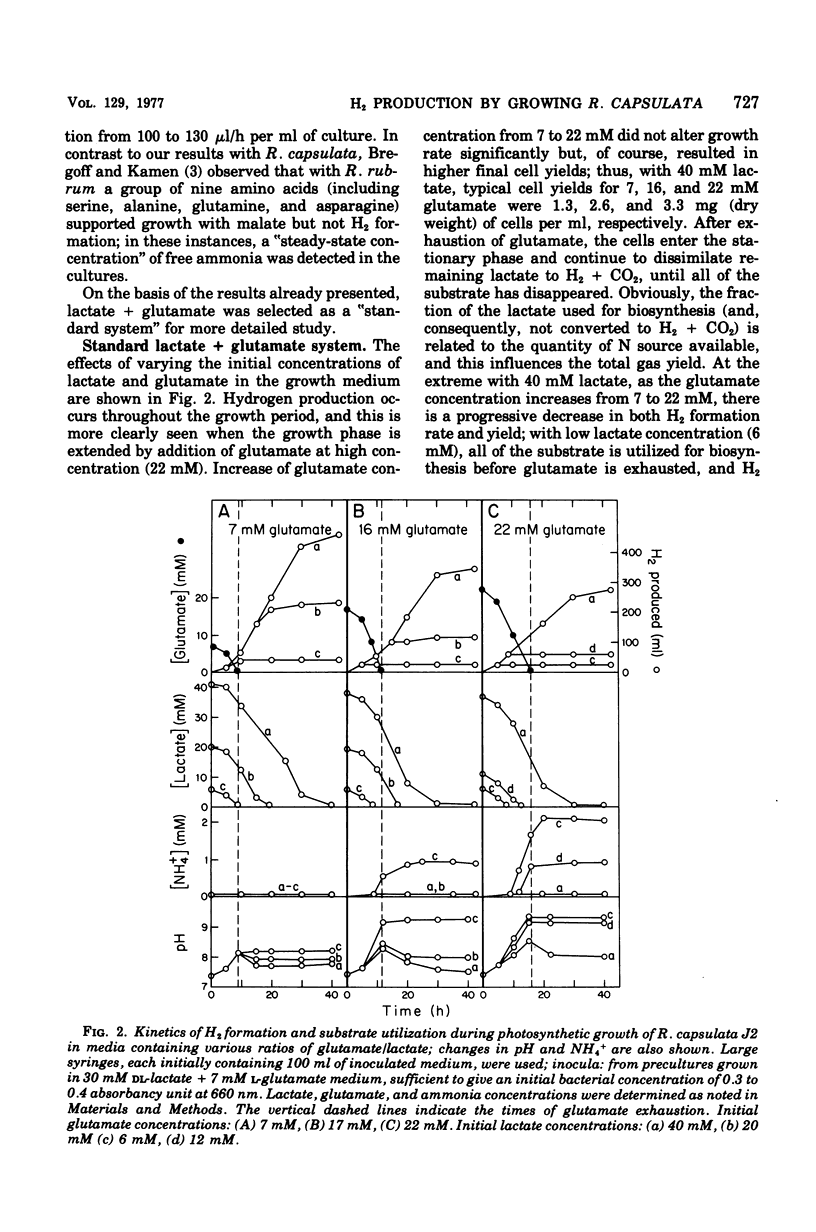
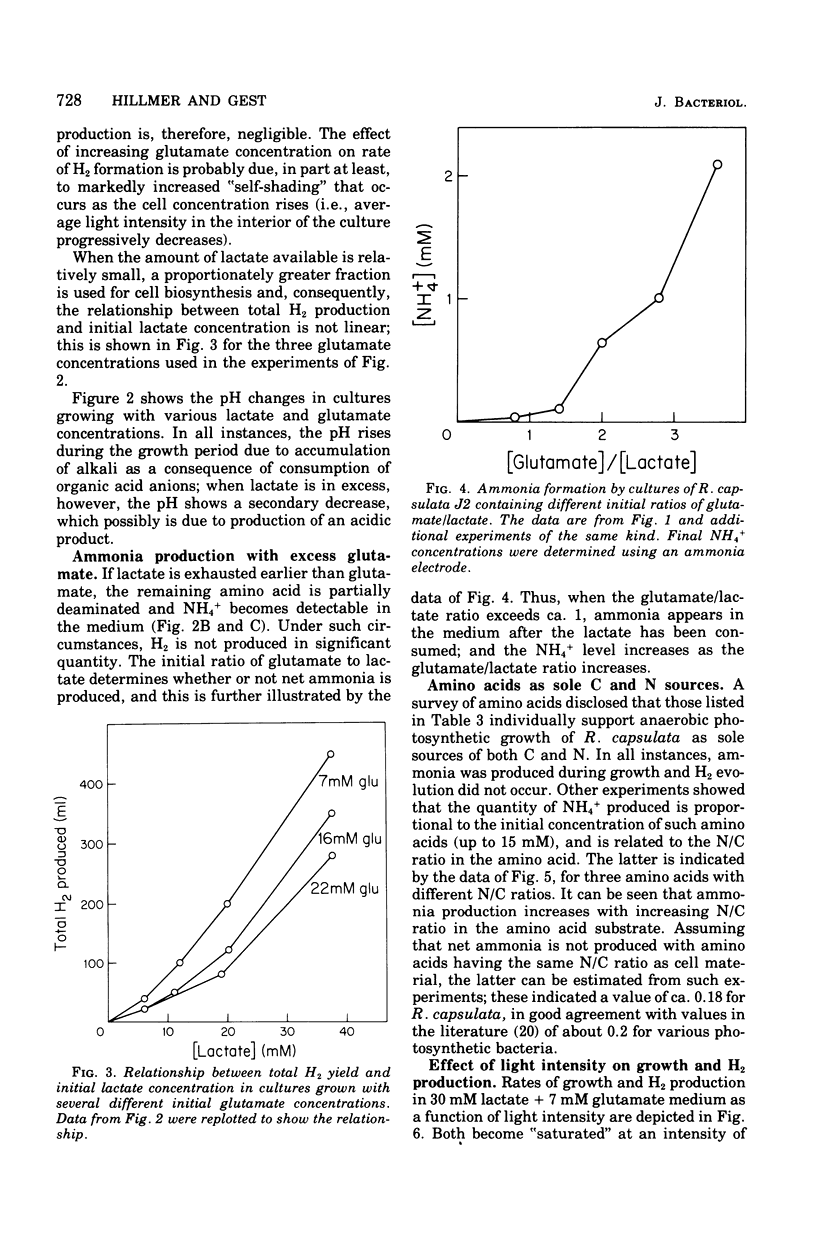
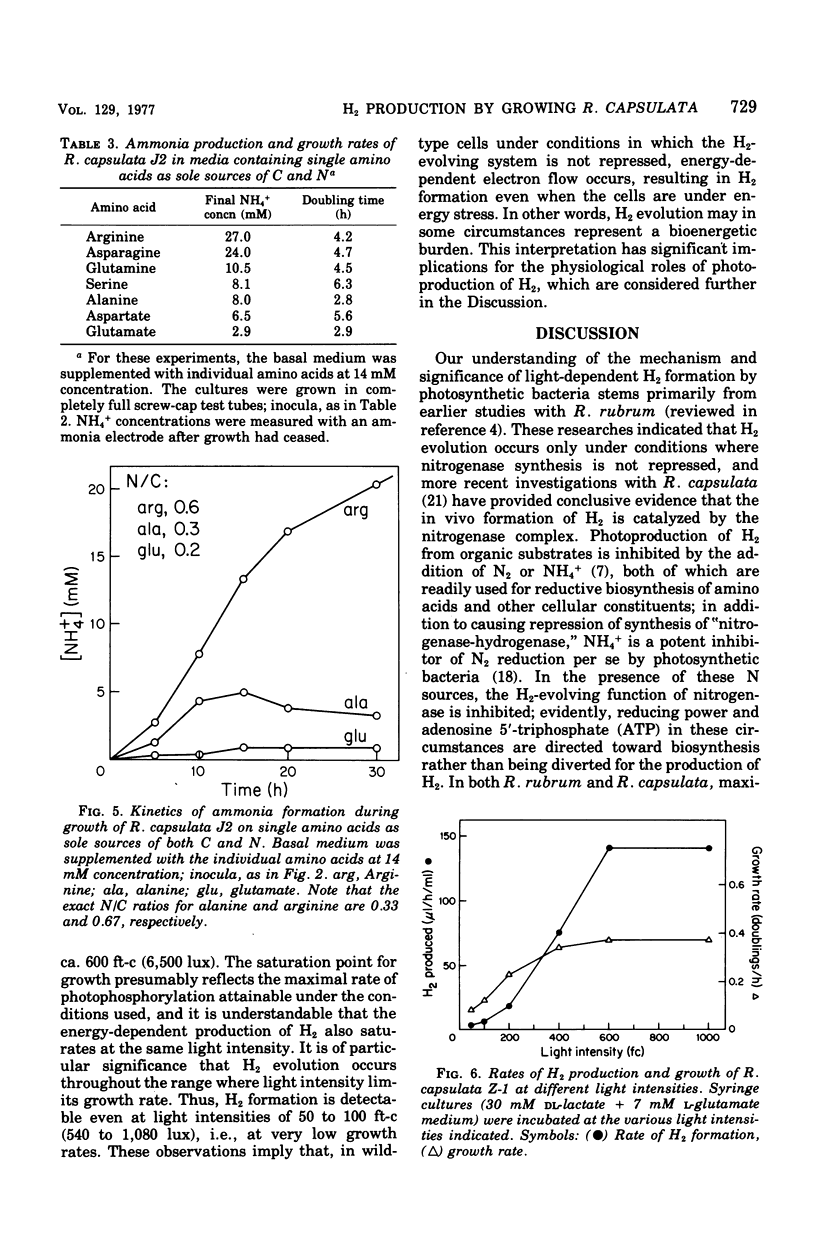
Abstract
Purple photosynthetic bacteria produce H2 from organic compounds by an anaerobic light-dependent electron transfer process in which nitrogenase functions as the terminal catalyst. It has been established that the H2-evolving function of nitrogenase is inhibited by N2 and ammonium salts, and is maximally expressed in cells growing photoheterotrophically with certain amino acids as sources of nitrogen. In the present studies with Rhodopseudomonas capsulata, nutritional factors affecting the rate and magnitude of H2 photoproduction in cultures growing with amino acid nitrogen sources were examined. The highest H2 yields and rates of formation were observed with the organic acids: lactate, pyruvate, malate, and succinate in media containing glutamate as the N source; under optimal conditions with excess lactate, H2 was produced at rates of ca. 130 ml/h per g(dry weight) of cells. Hydrogen production is significantly influenced by the N/C ratio in the growth substrates; when this ratio exceeds a critical value, free ammonia appears in the medium and H2 is not evolved. In the "standard" lactate + glutamate system, both H2 production and growth are "saturated" at a light intesity of ca. 600 ft-c (6,500 lux). Evolution of H2, however, occurs during growth at lithe intensities as low as 50 to 100 ft-c (540 to 1,080 lux), i.e., under conditions of energy limitation. In circumstances in which energy conversion rate and supplies of reducing power exceed the capacity of the biosynthetic machinery, energy-dependent H2 production presumably represents a regulatory device that facilitates "energy-idling." It appears that even when light intensity (energy) is limiting, a significant fraction of the available reducing power and adenosine 5'-triphosphate is diverted to nitrogenase, resulting in H2 formation and a bioenergetic burden to the cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREGOFF H. M., KAMEN M. D. Studies on the metabolism of photosynthetic bacteria. XIV. Quantitative relations between malate dissimilation, photoproduction of hydrogen, and nitrogen metabolism in Rhodospirillum rubrum. Arch Biochem Biophys. 1952 Mar;36(1):202–220. doi: 10.1016/0003-9861(52)90391-3. [DOI] [PubMed] [Google Scholar]
- Bregoff H. M., Kamen M. D. PHOTOHYDROGEN PRODUCTION IN CHROMATIUM. J Bacteriol. 1952 Jan;63(1):147–149. doi: 10.1128/jb.63.1.147-149.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H., ORMEROD J. G., ORMEROD K. S. Photometabolism of Rhodospirillum rubrum: light-dependent dissimilation of organic compounds to carbon dioxide and molecular hydrogen by an anaerobic citric acid cycle. Arch Biochem Biophys. 1962 Apr;97:21–33. doi: 10.1016/0003-9861(62)90039-5. [DOI] [PubMed] [Google Scholar]
- Gest H., Kamen M. D. Photoproduction of Molecular Hydrogen by Rhodospirillum rubrum. Science. 1949 Jun 3;109(2840):558–559. doi: 10.1126/science.109.2840.558. [DOI] [PubMed] [Google Scholar]
- Gest H., Kamen M. D. STUDIES ON THE METABOLISM OF PHOTOSYNTHETIC BACTERIA IV. : Photochemical Production of Molecular Hydrogen by Growing Cultures of Photosynthetic Bacteria. J Bacteriol. 1949 Aug;58(2):239–245. [PMC free article] [PubMed] [Google Scholar]
- Hillmer P., Gest H. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: production and utilization of H2 by resting cells. J Bacteriol. 1977 Feb;129(2):732–739. doi: 10.1128/jb.129.2.732-739.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen M. D., Gest H. Evidence for a Nitrogenase System in the Photosynthetic Bacterium Rhodospirillum rubrum. Science. 1949 Jun 3;109(2840):560–560. doi: 10.1126/science.109.2840.560. [DOI] [PubMed] [Google Scholar]
- Lueking D., Tokuhisa D., Sojka G. Glycerol assimilation by a mutant of Rhodopseudomonas capsulata. J Bacteriol. 1973 Sep;115(3):897–903. doi: 10.1128/jb.115.3.897-903.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON J. W., WILSON P. W. Nitrogen fixation and photoproduction of molecular hydrogen by Thiorhodaceae. Antonie Van Leeuwenhoek. 1953;19(1):71–77. doi: 10.1007/BF02594832. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., GEST H. Symposium on metabolism of inorganic compounds. IV. Hydrogen photosynthesis and alternative metabolic pathways in photosynthetic bacteria. Bacteriol Rev. 1962 Mar;26:51–66. doi: 10.1128/br.26.1.51-66.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- SIEGEL J. M., KAMEN M. D. Studies on the metabolism of photosynthetic bacteria. VII. Comparative studies on the photoproduction of H2 by Rhodo-pseudomonas gelatinosa and Rhodo-spirillum rubrum. J Bacteriol. 1951 Feb;61(2):215–228. doi: 10.1128/jb.61.2.215-228.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick H. J. Substrate and light dependent fixation of molecular nitrogen in Rhodospirillum rubrum. Arch Mikrobiol. 1971;75(2):89–101. doi: 10.1007/BF00407997. [DOI] [PubMed] [Google Scholar]
- Wall J. D., Weaver P. F., Gest H. Gene transfer agents, bacteriophages, and bacteriocins of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):217–224. doi: 10.1007/BF00447140. [DOI] [PubMed] [Google Scholar]
- Wall J. D., Weaver P. F., Gest H. Genetic transfer of nitrogenase-hydrogenase activity in Rhodopseudomonas capsulata. Nature. 1975 Dec 18;258(5536):630–631. doi: 10.1038/258630a0. [DOI] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Zilinsky J. W., Sojka G. A., Gest H. Energy charge regulation in photosynthetic bacteria. Biochem Biophys Res Commun. 1971 Mar 5;42(5):955–961. doi: 10.1016/0006-291x(71)90523-7. [DOI] [PubMed] [Google Scholar]



