Abstract
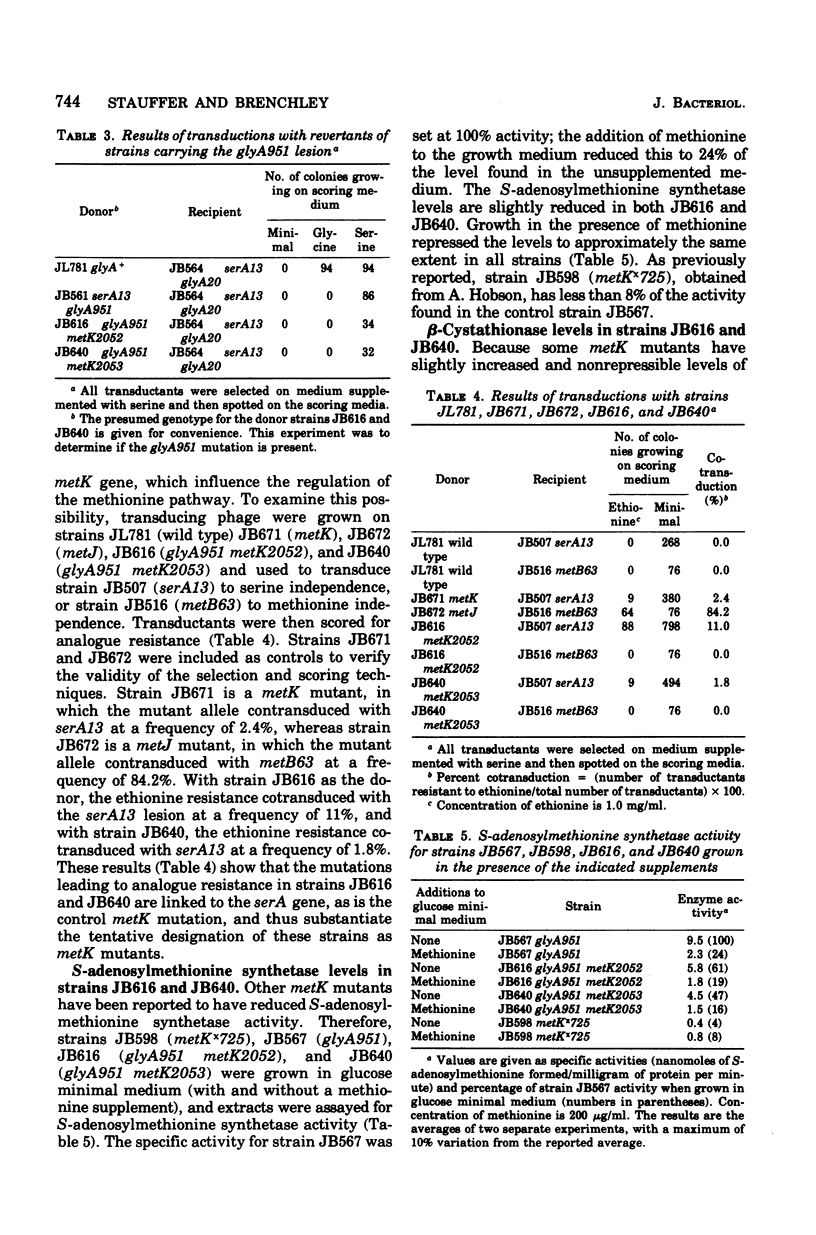
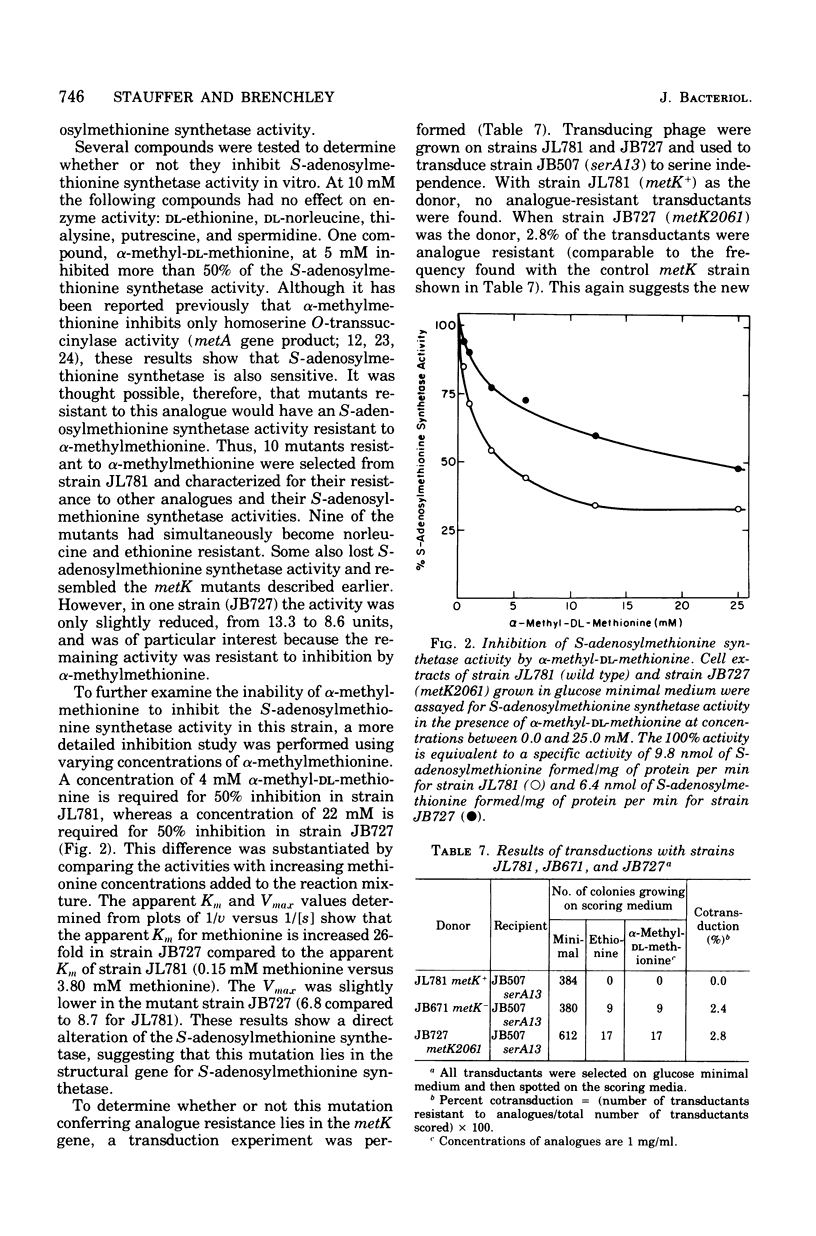
The enzyme serine transhydroxymethylase (EC 2.1.2.1; L-serine:tetrahydrofolate-5,10-hydroxymethyltransferase) is responsible both for the synthesis of glycine from serine and production of the 5,10-methylenetetrahydrofolate necessary as a methyl donor for methionine synthesis. Two mutants selected for alteration in serine transhydroxymethylase regulation also have phenotypes characteristic of metK (methionine regulatory) mutants, including ethionine, norleucine, and alpha-methylmethionine resistance and reduced levels of S-adenosylmethionine synthetase (EC 2.5.1.6; adenosine 5'-triphosphate:L-methionine S-adenosyltransferase) activity. Because this suggested the existence of a common regulatory component, the regulation of serine transhydroxymethylase was examined in other methionine regulatory mutants (metK and metJ mutants). Normally, serine transhydroxymethylase levels are repressed three- to sixfold in cells grown in the presence of serine, glycine, methionine, adenine, guanine, and thymine. This does not occur in metK and metJ mutants; thus, these mutations do affect the regulation of both serine transhydroxymethylase and the methionine biosynthetic enzymes. Lesions in the metK gene have been reported to reduce S-adenosylmethionine synthetase levels. To determine whether the metK gene actually encodes for S-adenosylmethionine synthetase, a mutant was characterized in which this enzyme has a 26-fold increased apparent Km for methionine. This mutation causes a phenotype associated with metK mutants and is cotransducible with the serA locus at the same frequency as metK lesions. Thus, the affect of metK mutations on the regulation of glycine and methionine synthesis in Salmonella typhimurium appears to be due to either an altered S-adenosylmethionine synthetase or altered S-adenosylmethionine pools.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. Mechanism of repression of methionine biosynthesis in Escherichia coli. I. The role of methionine, s-adenosylmethionine, and methionyl-transfer ribonucleic acid in repression. Mol Gen Genet. 1973 Jul 16;123(4):299–324. doi: 10.1007/BF00433648. [DOI] [PubMed] [Google Scholar]
- Aswad D. W., Koshland D. E., Jr Evidence for an S-adenosylmethionine requirement in the chemotactic behavior of Salmonella typhimurium. J Mol Biol. 1975 Sep 15;97(2):207–223. doi: 10.1016/s0022-2836(75)80035-0. [DOI] [PubMed] [Google Scholar]
- Boro H., Brenchley J. E. A new generalized transducing phage for Salmonella typhimurium LT2. Virology. 1971 Sep;45(3):835–836. doi: 10.1016/0042-6822(71)90208-x. [DOI] [PubMed] [Google Scholar]
- Brenchley J. E. Effect of methionine sulfoximine and methionine sulfone on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973 May;114(2):666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Radovich C. Role of methionine in the regulation of serine hydroxymethyltransferase in Eschericia coli. J Bacteriol. 1975 Oct;124(1):269–278. doi: 10.1128/jb.124.1.269-278.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson A. C., Smith D. A. S-adenosylmethionine synthetase in methionine regulatory mutants of Salmonella typhimurium. Mol Gen Genet. 1973 Oct 16;126(1):7–18. doi: 10.1007/BF00333477. [DOI] [PubMed] [Google Scholar]
- Hobson A. C. The regulation of methionine and s-adenosylmethionine biosynthesis and utilization in mutants of Salmonella typhimurium with defects in s-adenosylmethionine synthetase. Mol Gen Genet. 1974;131(3):263–273. doi: 10.1007/BF00267965. [DOI] [PubMed] [Google Scholar]
- Hobson A. C. The synthesis of S-adenosylmethionine by mutants with defects in S-adenosylmethionine synthetase. Mol Gen Genet. 1976 Feb 27;144(1):87–95. doi: 10.1007/BF00277310. [DOI] [PubMed] [Google Scholar]
- Holloway C. T., Greene R. C., Su C. H. Regulation of S-adenosylmethionine synthetase in Escherichia coli. J Bacteriol. 1970 Nov;104(2):734–747. doi: 10.1128/jb.104.2.734-747.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence D. A. Regulation of the methionine feedback-sensitive enzyme in mutants of Salmonella typhimurium. J Bacteriol. 1972 Jan;109(1):8–11. doi: 10.1128/jb.109.1.8-11.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A., Smith D. A., Rowbury R. J. Regulation of methionine synthesis in Salmonella typhimurium: mutants resistant to inhibition by analogues of methionine. Genetics. 1968 Apr;58(4):473–492. doi: 10.1093/genetics/58.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A., Decter J. B., Silber R. Studies on the regulation of one-carbon metabolism. II. Repression-derepression of serine hydroxymethyltransferase by methionine in Escherichia coli 113-3. J Biol Chem. 1972 Jan 25;247(2):348–352. [PubMed] [Google Scholar]
- McIntire S. A. Transduction with integration-defective mutants of Salmonella typhimurium bacteriophage KB1. J Bacteriol. 1974 Feb;117(2):907–908. doi: 10.1128/jb.117.2.907-908.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meedel T. H., Pizer L. I. Regulation of one-carbon biosynthesis and utilization in Escherichia coli. J Bacteriol. 1974 Jun;118(3):905–910. doi: 10.1128/jb.118.3.905-910.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. A., Newman E. B. Control of serine transhydroxymethylase synthesis in Escherichia coli K12. Can J Microbiol. 1974 Jan;20(1):41–47. doi: 10.1139/m74-007. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Taya Y., Kuchino Y., Oashi Z. Enzymatic synthesis of 3-(3-amino-3-carboxypropyl)uridine in Escherichia coli phenylalanine transfer RNA: transfer of the 3-amino-acid-3-carboxypropyl group from S-adenosylmethionine. Biochem Biophys Res Commun. 1974 Apr 8;57(3):702–708. doi: 10.1016/0006-291x(74)90603-2. [DOI] [PubMed] [Google Scholar]
- PIZER L. I. GLYCINE SYNTHESIS AND METABOLISM IN ESCHERICHIA COLI. J Bacteriol. 1965 Apr;89:1145–1150. doi: 10.1128/jb.89.4.1145-1150.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I., POTOCHNY M. L. NUTRITIONAL AND REGULATORY ASPECTS OF SERINE METABOLISM IN ESCHERICHIA COLI. J Bacteriol. 1964 Sep;88:611–619. doi: 10.1128/jb.88.3.611-619.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rej R., Richards A. H. Interference by Tris buffer in the estimation of protein by the Lowry procedure. Anal Biochem. 1974 Nov;62(1):240–247. doi: 10.1016/0003-2697(74)90383-2. [DOI] [PubMed] [Google Scholar]
- Schlesinger S. Inhibition of growth of Escherichia coli and of homoserine O-transsuccinylase by alpha-methylmethionine. J Bacteriol. 1967 Aug;94(2):327–332. doi: 10.1128/jb.94.2.327-332.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer G. V., Baker C. A., Brenchley J. E. Regulation of serine transhydroxymethylase activity in Salmonella typhimurium. J Bacteriol. 1974 Dec;120(3):1017–1025. doi: 10.1128/jb.120.3.1017-1025.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer G. V., Brenchley J. E. Evidence for the involvement of serine transhydroxymethylase in serine and glycine interconversions in Salmonella typhimurium. Genetics. 1974 Jun;77(2):185–198. doi: 10.1093/genetics/77.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner G. L., Eisenberg M. A. Purification and properties of 7, 8-diaminopelargonic acid aminotransferase. J Biol Chem. 1975 Jun 10;250(11):4029–4036. [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- Taylor R. T., Dickerman H., Weissbach H. Control of one-carbon metabolism in a methionine-B12 auxotroph of Escherichia coli. Arch Biochem Biophys. 1966 Nov;117(2):405–412. doi: 10.1016/0003-9861(66)90429-2. [DOI] [PubMed] [Google Scholar]


