Abstract
Human β2-glycoprotein I (β2GPI) binds to recombinant hepatitis B surface antigen (rHBsAg), but the location of the binding domain on β2GPI is unknown. It has been suggested that the lipid rather than the protein moiety of rHBsAg binds to β2GPI. Since β2 GPI binds to anionic phospholipids (PL) through its lipid binding region in the fifth domain of β2GPI, we predicted that this lipid binding region may also be involved in binding rHBsAg. In this study, we examined rHBsAg binding to two naturally occurring mutants of β2GPI, Cys306Gly and Trp316Ser, or evolutionarily conserved hydrophobic amino acid sequence, Leu313-Ala314-Phe315 in the fifth domain of β2GPI. The two naturally occurring mutations and two mutagenized amino acids, Leu313Gly or Phe315Ser, disrupted the binding of recombinant β2GPI (rβ2GPI) to both rHBsAg and cardiolipin (CL), an anionic PL. These results suggest that rHBsAg and CL share the same region in the fifth domain of β2GPI. Credence to this conclusion was further provided by competitive ELISA, where CL-bound rβ2GPI was incubated with increasing amounts of rHBsAg. As expected, pre-incubation of rβ2GPI with CL precluded binding to rHBsAg, indicating that CL and rHBsAg bind to the same region on β2GPI. Our data provide evidence that the lipid (PL) rather than the protein moiety of rHBsAg binds to β2GPI and that this binding region is located in the fifth domain of β2GPI, which also binds to anionic PL.
Keywords: Hepatitis B surface antigen, β2-Glycoprotein I, Apolipoprotein H, Anionic phospholipids
1. Introduction
Hepatitis B virus (HBV) belongs to a DNA-containing small enveloped hepadanavirus family, which includes woodchuck hepatitis virus [1], ground squirrel hepatitis virus [2], duck hepatitis B virus [3], and heron hepatitis virus [4]. These viruses infect and replicate primarily in hepatocytes in vivo and often lead to chronic infection in the cells of their respective hosts [5]. However, the mode of entry for HBV into the human hepatocytes or the HBV receptor is not well established. Several potential candidates for HBV receptor have been reported, including a 50 kDa glycoprotein known as β2-glycoprotein I (β2GPI) [6,7]. β2GPI, also known as apolipoprotein H, is a plasma glycoprotein [8], which is associated with very low-density lipoproteins (VLDL), high-density lipoproteins (HDL), low-density lipoprotein (LDL) and chylomicrons, and it also exists in lipid-free form in plasma [9,10]. Furthermore, β2GPI shows higher binding with oxidized LDL than native LDL [11–13]. Mature β2GPI is a single chain polypeptide of 326 amino acids [14–17] and belongs to the short consensus repeats (SCR) or complement control protein (CCP) superfamily consisting of five homologous repeats (~60 amino acids each) referred to as: GP-I domains, Sushi domains, SCRs or SSP repeats. There are 22 cystine residues in human β2GPI, which are conserved in bovine, rat, mouse and dog [18–21]. The first four GP-I domains (~60 amino acids each) are structurally related, while the fifth domain (84 amino acids) is the most variable and includes a cluster of lysine residues (282–287), four highly conserved hydrophobic amino acids (313–316), and three disulfide bonds instead of two as in each of the preceding four domains. Previously, we demonstrated that the β2GPI cDNA transfected COS-1 cells expressed and secreted full-length recombinant β2GPI (rβ2GPI) into its culture medium that exhibits normal binding to recombinant hepatitis B surface antigen (rHBsAg) [6]. We have also demonstrated that carbohydrate side chains and arginine residues of β2GPI have no effect on its ability to bind to rHBsAg, but this binding was disrupted by the reduction of disulfide bonds and by the chemical modification of as few as three lysine residues [7]. Since the fifth domain of β2GPI has a cluster of lysine residues (282–287) and three disulfide bonds, it was predicted to contain the binding site for rHBsAg.
Using either artificial mixtures of lipids [10] or pure lipids [22] it was originally suggested that ionic and hydrophobic interactions play major roles in the binding of β2GPI with phospholipids (PL). The binding of β2GPI with anionic PL triggers the production of a subset antiphospholipid antibodies (APA) in autoimmune diseases [23,24]. Several natural mutations have been reported in the β2GPI gene, including Ser88Asn, Val247Leu, Cys306Gly, and Trp316Ser [25–27]. The two mutations in the fifth domain of β2GPI, Cys306Gly and Trp316Ser, may be important because Cys306Gly disrupts one of the disulfide bonds and Trp316Ser disrupts the integrity of a cluster of hydrophobic amino acids at positions 313–316 (Leu-Ala-Phe-Trp), which are evolutionarily conserved in bovine, rat, mouse, dog, and human β2GPI [18–21]. Previously, we demonstrated that the Cys306Gly and Trp316Ser mutations disrupt the binding of plasma β2GPI [27] and rβ2GPI [28] to anionic PL. By site-directed mutagenesis, we have also shown that in addition to Trp316, Leu313 and Phe315 are required for rβ2GPI-PL binding [28].
Since the binding of β2GPI to rHBsAg is also dependent on the integrity of disulfide bond(s) and some lysine residues whose locations are not known [6,7], we hypothesized that rHBsAg would bind to the same region in the fifth domain of β2GPI that is involved in binding anionic PL. To examine this hypothesis, we mutagenized the fifth domain of β2GPI and determined the effect of these mutations on its ability to bind rHBsAg compared to PL. We also performed a competition assay in an attempt to discriminate between the binding of rHBsAg and anionic PL to rβ2GPI. Our results show that rHBsAg and anionic PL compete for the same binding region on rβ2GPI.
2. Materials and Methods
2.1. Site-Directed Mutagenesis
Previously described cloned β2GPI cDNA in eukaryotic expression vector (pRc/CMV, Invitrogen) [16] was used to create the desired mutations using the QuickChange Site-Directed Mutagenesis kit (Strategene) as described earlier [28]. Briefly, two mutagenic primers containing the desired mutation in the middle were used to PCR amplify the entire recombinant plasmid with Pfu DNA polymerase to mutate the β2GPI cDNA. For each mutagenesis experiments, four to eight colonies were grown in 5 ml of LB media containing ampicillin at 37°C for 16 h. The plasmid DNA was extracted by the Spin Plasmid Miniprep kit (Qiagen) and sequenced by chain-termination dideoxynucleosides methods [29] using Sequenase Version 2.0 DNA Sequencing kit (United States Biochemical) to confirm the mutation as well as the fidelity of Pfu DNA polymerase.
2.2. Transient Expression of rβ2GPI
COS-1 cells were maintained as a monolayer in Dulbecco's Modified Eagle Medium (DMEM) (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS) (HyClone), 2 mM glutamine, 100 u/ml penicillin, and 100 μg/ml streptomycin at 37°C under 5% CO2. Subconfluent monolayer of the cells grown overnight in 60 mm dishes were transiently transfected with wild type and mutant type β2GPI cDNA by DEAE-dextran method as described earlier [28, 30]. After 48 hrs of transfection, cells were washed twice with phosphate-buffer-saline (PBS) and incubated for an additional 4–12 h in serum-free culture media to collect the secreted rβ2GPI.
2.3. Detection of rβ2GPI by Capture ELISA
The rβ2GPl levels were detected by modified capture ELISA [6]. Briefly, 50 μl of anti-β2GPI monoclonal antibody (mAb) (Chemicon International, Inc.) (5–7 μg/ml in PBS) was adsorbed onto 96 well vinyl microtiter plates (Costar) by overnight incubation at 4°C with a cover to prevent evaporation. The wells were washed three times with PBS and blocked with PBS containing 1 % bovine serum albumin (BSA) at 37°C for 90 minutes. Fifty μI of serum-free-cell-free culture media from the transfected COS-1 cells containing rβ2GPI was added to the mAb-coated wells in triplicate followed by polyclonal rabbit anti-β2GPI antiserum (Alexis Corp.) (0.5 μg/ml in PBS containing 1% BSA) and then by alkaline phosphatase-conjugated goat anti-rabbit IgG antiserum (Instar Corp.). Each reaction was incubated at 37°C for 90 minutes followed by addition of 100 μI of para-nitrophenyl phosphate (PNP) substrate (Chemicon International, Inc.). The wells were washed three times with PBS between each incubation for all binding assays. The color intensity was measured as optical density (OD) at 410 nm after different time intervals.
2.4. Binding of rβ2GPI to rHBsAg
The rβ2GPI captured on anti-β2GPI mAb coated wells were incubated with 2.5 μg of rHBsAg (rS,L* particles was produced in Saccharomyces cerevisiae [31,32]) diluted in PBS containing 1% BSA at 22°C for 16 hrs [6]. The bound rHBsAg was then detected by goat anti-hepatitis B surface antigen (anti-HBs) antiserum (OAKO Corp.) followed by alkaline phosphatase-conjugated rabbit anti-goat IgG antiserum (Instar Corp.). Each reaction was incubated at 37°C for 90 minutes followed by addition of 100 μI of PNP. The wells were washed three times with PBS between each incubation for all binding assays. The color intensity was measured as optical density (OD) at 410 nm after different time intervals.
2.5. Binding of rβ2GPI to Cardiolipin (CL)
The binding of rβ2GPI to CL was performed by CL-ELISA as previously described [28]. Briefly, 96 well vinyl microtiter plates (Costar) were coated with 30 μI of CL solution in ethanol (50 μg/ml) (Sigma). The plates were dried under vacuum and blocked with PBS containing 1% milk and 0.3% gelatin at 25°C for 1 hr. The plates were washed three times with PBS and incubated with 50 μI of serum-free-cell-free culture media of transfected COS-1 cells containing rβ2GPI at 25°C for 3 hrs. The bound rβ2GPI was detected by polyclonal rabbit anti-β2GPI antiserum (Alexis Corp.) (0.5 μg/ml in PBS containing 1% BSA) followed by alkaline phosphatase conjugated goat anti-rabbit IgG antiserum (Instar Corp.). Each reaction was incubated at 37°C for 90 minutes followed by addition of 100 μI of PNP. The color intensity was measured as optical density (OD) at 410 nm after different time intervals.
2.6. Competition Assays
A competition assay was performed in which CL coated microtiter wells were incubated with 50 μI of serum-free-cell-free culture media from transfected COS-1 cells containing rβ2GPI at 25°C for 3 hrs followed by three washings with PBS. The CL-bound rβ2GPI was then allowed to bind to the increasing amount of rHBsAg (rS,L* particles, 0.31 to 10 μg/well diluted in PBS containing 1% BSA) and the bound rHBsAg was detected by anti-HBs antiserum (DAKO Corp.) as described above. Control wells received no rHBsAg. The CL-bound rβ2GPI was detected with polyclonal rabbit anti-β2GPI antiserum, as described above. A control assay was also performed where rβ2GPI captured by anti-β2GPI mAb coated microtiter wells were allowed to bind to increasing amounts of rHBsAg (rS,L* particles, 0.31 to 10 μg/well diluted in PBS containing 1% BSA) and the bound rHBsAg was detected by anti-HBs antiserum, as described above.
3. Results
3.1. Effect of the Cys306Gly and Trp316Ser Mutations on rHBsAg Binding
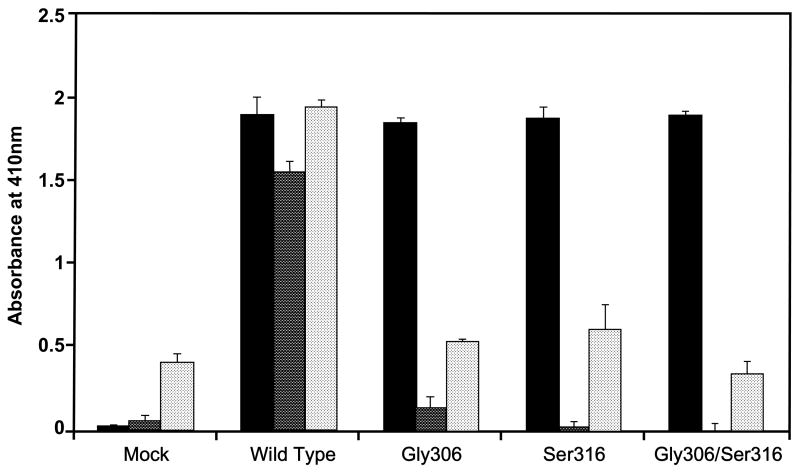
Figure 1 shows the secreted levels of wild-type and mutant rβ2GPI into the serum-free-cell-free culture media of the transfected COS-1 cells and the binding of rβ2GPI wild and mutant types to rHBsAg or CL. While the levels of wild-type and mutant rβ2GPI secreted into the culture media were comparable (solid bars), the binding of rβ2GPI mutants (Gly306, Ser316, and Gly306/Ser316) to rHBsAg were significantly reduced as compared to the wild-type rβ2GPI (dark bars). The isoform-specific binding of rβ2GPI with rHBsAg (dark bars) was similar to that observed for the CL-rβ2GPI binding (light bars). These results show that a single mutation either at codon 306 or 316 in the fifth domain of β2GPI is sufficient to disrupt the interaction of rβ2GPI with either rHBsAg or CL.
Fig. 1.
Effect of naturally occurring missense mutations in the fifth domain of β2GPI (Cys306Gly and Trp316Ser) on its ability to bind to rHBsAg and CL. The rβ2GPI secreted into the culture medium of mock, wild-type, Gly306 mutant, Ser316 mutant and Gly306/Ser316 double mutant transfected COS-1 cells was quantitated by capture ELISA (■). The secreted wild-type and mutant-type rβ2GPI were then allowed to bind to rHBsAg (
 ) or CL (
) or CL (
 ) on microtiter plates to examine the effect of these mutations on the ability of rβ2GPI to bind to rHBsAg or CL. Results are given as mean ± SD from three independent clones in triplicate (n=9).
) on microtiter plates to examine the effect of these mutations on the ability of rβ2GPI to bind to rHBsAg or CL. Results are given as mean ± SD from three independent clones in triplicate (n=9).
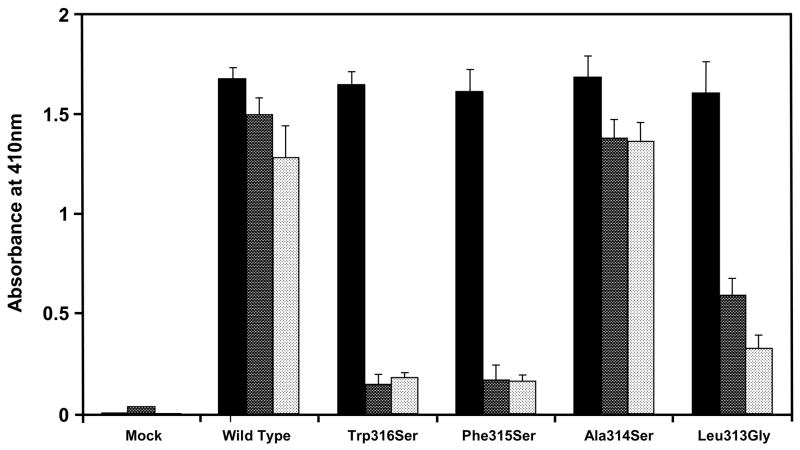
3.2. Effect of Hydrophobic Amino Acids at Positions 313–316 on rHBsAg Binding
In addition to the naturally occurring Trp316Ser mutation, we changed the remaining three conserved hydrophobic amino acids at positions 313–315 to neutral amino acids (Leu313Gly, Ala314Ser and Phe315Ser) in β2GPI cDNA by site-directed mutagenesis, expressed them in COS-1 cells, and examined the binding of the mutant-type rβ2GPI with rHBsAg or CL (Fig. 2). While Ala314Ser had no effect on the binding of rβ2GPI with rHBsAg (92%; dark bars) or CL (106%; light bars), the Leu313Gly and Phe315Ser mutations significantly reduced the binding of mutant rβ2GPI with rHBsAg (38% and 9% of the wild-type rβ2GPI, respectively; dark bars) as well as with CL (25% and 13% of the wild-type rβ2GPI, respectively; light bars). However, the levels of rβ2GPI secreted into the serum-free-cell-free culture media of the wild-type and each mutant β2GPI cDNA transfected COS-1 cells were comparable (solid bars). The results from these experiments further demonstrate that rHBsAg and CL share same binding region on the β2GPI molecules.
Fig. 2.
Effect of hydrophobic amino acids in the fifth domain of rβ2GPI at positions 313–316 on its ability to bind to rHBsAg and CL. Four hydrophobic amino acids at positions 313–316 were mutated to neutral amino acids (Trp316Ser, Phe315Ser, Ala314Ser and Leu313Gly) and expressed in COS-1 cells. The secreted rβ2GPI forms were quantitated by capture ELISA (■) followed by their binding to rHBsAg (
 ) or CL (
) or CL (
 ). Results are given as mean ± SD from three independent clones in triplicate (n=9).
). Results are given as mean ± SD from three independent clones in triplicate (n=9).
3.3. Competition Between rHBsAg and CL for Binding to β2GPI
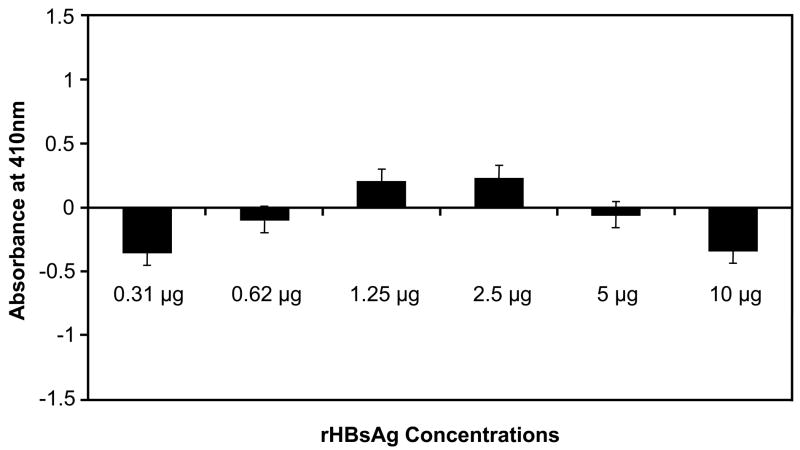
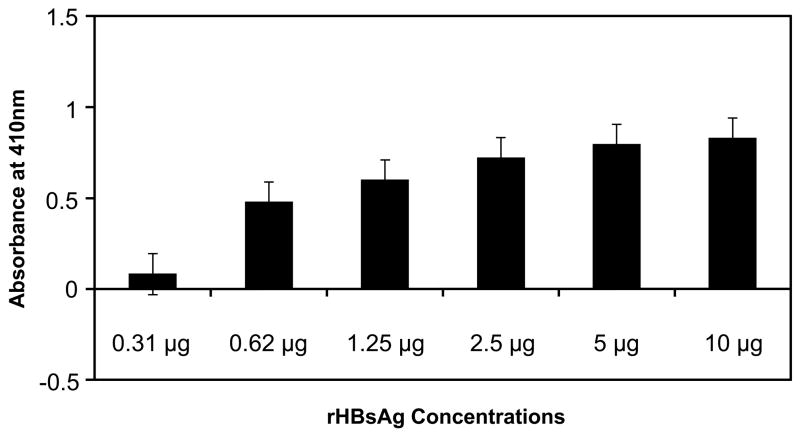
To confirm our findings that rHBsAg and CL bind to the same region on β2GPI, we performed two competition assays. In the first assay, CL-bound rβ2GPI on microtiter wells was allowed to bind with an increasing amount of rHBsAg (0.31 to 10 μg/well) (Fig. 3A). As expected, CL-bound rβ2GPI exhibited no binding with rHBsAg, as the binding site for rHBsAg on β2GPI was blocked by CL. These results suggest that the binding of rB2GPI to CL is saturated with higher affinity than with rHBsAg because the addition of increasing amounts of rHBsAg did not affect rβ2GPI-CL binding. A second assay was designed to test whether steric hindrance or steric resistance is the reason for lack of binding of rHBsAg to rβ2GPI-CL complex. In this experiment, rβ2GPI captured by anti-β2GPI mAb on microtiter wells was incubated with increasing amounts of rHBsAg (Fig. 3B). The rHBsAg binding to the mAb-captured rβ2GPI increased with increasing amount of rHBsAg added (Fig. 3B) as opposed to CL bound rβ2GPI, which demonstrated no binding to rHBsAg even at highest concentration (Fig. 3A). This suggests that the binding sites for rHBsAg (or CL) and mAb on β2GPI are different and thus steric hindrance caused by the mAb is not a factor.
Fig. 3. Competition assay.
(A) Microtiter wells coated with CL were allowed to bind to rβ2GPI and then incubated for 16 hrs with increasing amount rHBsAg. (B) Microtiter wells coated with anti-β2GPI were allowed to bind to rβ2GPI followed by binding to increasing amount rHBsAg. Results are given as mean ± SD from three independent experiments in triplicate (n=9).
4. Discussion
The mechanism of HBV entry and its cellular receptor on the surface of human hepatocytes are not well understood. However, several candidate receptors have been identified that includes β2GPI, which was first reported by us [6,7] and latter by others [33–35]. β2GPI is a secretary glycoprotein that is present in plasma in association with several lipoprotein particles as well as in lipid-free form [9,10,22]. Since β2GPI is not a transmemebrane protein, a typical criterion for a viral receptor, we have earlier hypothesized that β2GPI present on the surface of lipoprotein particles might serve as a receptor for HBV and that the lipoprotein-HBV complex enters hepatocytes during the process of lipoprotein uptakes [6]. Although the experimental data supporting this hypothesis is lacking at present, here we have addressed several critical questions regarding the interaction between β2GPI and HBV.
Previously, we demonstrated that the binding of β2GPI with rHBsAg was disrupted by reducing the intramolecular disulfide bonds of β2GPI [6] and chemical modification of as few as three positively charged lysine residues of β2GPI [7]. However, this interaction was not affected by the removal of β2GPI carbohydrate side chains or chemical modification of arginine, the other positively charged amino acid [7]. These results suggested that the interaction between β2GPI and rHBsAg was most likely due to protein-protein interaction. On the other hand, Neurath and Strick [36] have suggested that since delipidation of rHBsAg abolishes β2GP1-rHBsAg binding, that the lipid moiety of rHBsAg is involved in binding β2GPI. Subsequently, Stefas et al. [35] have demonstrated that the myristylated pre-S1 domain of HBsAg strongly interacted with β2GPI, which involved PL component of HBV envelope because the removal of PL component by detergent or oxidation prevented β2GPI-HBsAg interaction. Notwithstanding whether it is the lipid or protein moiety of rHBsAg that binds to β2GPI, the β2GPI domain that binds to rHBsAg is not known.
A robust way to localize the binding domain of β2GPI is to assess the role of naturally occurring or artificially created missense mutations of β2GPI in binding rHBsAg. We identified two naturally occurring missense mutations, Cys306Gly and Trp316Ser in the fifth domain of β2GPI that disrupt PL binding to β2GPI [27,28]. The Cys306Gly mutation disrupts a disulfide bond between Cys306 and Cys281, which is critical for clustering several lysine residues [15]. The Trp316Ser mutation disrupts the integrity of an evolutionarily conserved hydrophobic amino acid sequence at positions 313–316 [15]. We hypothesized that these mutations would also disrupt β2GPI-rHBsAg binding. Indeed our data demonstrate that the mutant rβ2GPI carrying one or both mutations do not bind to rHBsAg. These results show that rHBsAg and anionic PL share the same binding region on the β2GPI molecule. To further identify or discriminate between the binding sites of rHBsAg and anionic PL on β2GPI, we examined the effect of the remaining evolutionarily conserved hydrophobic amino acids in the vicinity of the Trp316Ser mutation at positions 313–315 on the ability of β2GPI to bind rHBsAg. Three hydrophobic amino acids at positions 313–315 were mutated to neutral residues (Leu313Gly, Ala314Ser and Phe315Ser) and the mutant rβ2GPI were then allowed to bind rHBsAg or CL. The Leu313Gly and Phe315Ser mutations significantly reduced the binding of mutant rβ2GPI to both rHBsAg and CL, while the Ala314Ser mutation showed normal binding with both rHBsAg and CL. These results indicate that the binding of β2GPI to rHBsAg or CL involves both ionic (shown by the Cys306Gly mutation, which disrupts the clustering of lysine residues of the fifth domain) and hydrophobic (shown by the mutagenized 313–316 sequence) interactions.
Further evidence that rHBsAg and CL share a binding region on β2GPI comes from our competition assay in which rβ2GPI bound to CL-coated microtiter wells was allowed to bind to increasing amounts of rHBsAg. If CL and rHBsAg share same binding region on rβ2GPI then CL-bound rβ2GPI would not be able to bind rHBsAg. Indeed, we found that rβ2GPI bound to CL was unable to bind rHBsAg. This suggests that rHBsAg and CL compete for the same binding region on rβ2GPI. Although it is possible that the rβ2GPI bound to the well via CL might be in the correct orientation to bind to rHBsAg if the two binding regions on rβ2GPI were different as in case of anti-β2GPI antibodies (the mAb) which did bind to CL-bound rβ2GPI.
Our results on rHBsAg binding with β2GPI using the naturally occurring mutations as well as artificially created mutations in the lipid-binding region of β2GPI suggest that the lipid (PL) rather than the protein moiety of rHBsAg binds to rβ2GPI, as originally suggested by Neurath and Strick [36] and latter by Stefas et al. [35]. The Cys306Gly mutation in the fifth domain of β2GPI disrupts the clustering of lysine residues, which are required for ionic binding with PL. Likewise, the fifth domain is necessary for the hydrophobic binding with PL [37–39]. Since mutations at this region also disrupt the binding of β2GPI with rHBsAg, it appears that the lipid moiety, perhaps anionic PL, rather than the protein moiety of rHBsAg, is involved in binding with β2GPI. Indirect support to the hypothesis that the lipid moiety of rHBsAg binds to the lipid-binding region in the fifth domain of β2GPI comes from another competition assay where the rβ2GPI captured by anti-β2GPI mAb on coated microtiter wells was able to bind to rHBsAg. This indicates that while the lipid moiety of rHBsAg binds to the lipid-binding region in the fifth domain of β2GPI, the commercially available anti-β2GPI mAb binds to a different domain of β2GPI but the rβ2GPI-CL complex could not bind rHBsAg confirming that the CL and rHBsAg bind to the same region on rβ2GPI.
In summary, we have identified the binding region for rHBsAg on β2GPI, which is located in the fifth domain of β2GPI and it appears to be the same as PL-binding region on β2GPI. Our results in conjunction with the observation of Neurath and Strick [36] and Stefas et al., [35] indicate that rHBsAg binds to β2GPI through its lipid moiety. These results also confirm our previous reports that the interaction between rHBsAg and β2GPI is strong and saturable [6,7].
Acknowledgments
We thank Dr. Tineke Rutgers, Dmith Kline-Beachem for the rHBsAg and Dr. Mark E. Peeples, Rush University for providing β2GPI cDNA and critically reviewing our manuscript. This work was supported by a National Heart, Lung, and Blood Institute (NHLBI) grant HL 54900.
Abbreviation
- HBV
Hepatitis B virus
- HBsAg
Hepatitis B surface antigen
- r
Recombinant
- PL
Phospholipid
- β2GPI
β2-Glycoprotein I
- apoH
Apolipoprotein H
- APA
Antiphospholipid antibodies
- CL
Cardiolipin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Summers J, Smolec J, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchuck. Proc Natl Acad Sci USA. 1978;75:4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marion PL, Oshiro LS, ReQnery DC, Scullard GH, Robinson WS. A virus in Beechey ground squirrels which is related to hepatitis B virus of man. Proc Natl Acad Sci USA. 1980;77:2941–2945. doi: 10.1073/pnas.77.5.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason WS, Seal S, Summers J. Virus Peking ducks with structurally and biologically relatedness to human hepatitis B virus. J Virol. 1980;36:829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprengel R, Kaleta EF, Will H. Isolation and characterization of a hepatitis 8 virus endemic in herons. J Virol. 1988;52:3832–3839. doi: 10.1128/jvi.62.10.3832-3839.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summers J. Three recently described animal virus models for human hepatitis B virus. Hepatology. 1981;1:179–183. doi: 10.1002/hep.1840010215. [DOI] [PubMed] [Google Scholar]
- 6.Mehdi H, Kaplan MJ, Anlar FY, Yang X, Bayer R, Sutherland K, Peeples ME. Hepatitis B virus surface antigen binds to apolipoprotein H. J Virol. 1994;68:2415–2424. doi: 10.1128/jvi.68.4.2415-2424.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehdi H, Yang X, Peeples ME. An altered form of apolipoprotein H binds hepatitis B virus surface antigen most efficiently. Virology. 1996;217:58–66. doi: 10.1006/viro.1996.0093. [DOI] [PubMed] [Google Scholar]
- 8.Schultz HE, Heide K, Haupt H. Uber ein bisher unbekanntes neidermolekul es β2-Globulin des Humanserums. Naturwissenschaften. 1961;48:719. [Google Scholar]
- 9.Polz E, Kostner GM. The binding of β2-glycoprotein I to human serum lipoproteins: Distribution among density fractions. FEBS Lett. 1979;102:183–186. doi: 10.1016/0014-5793(79)80955-2. [DOI] [PubMed] [Google Scholar]
- 10.Polz E, Kostner GM. Binding of β2-glycoprotein I to intralipid: Distribution of the dissociation constant. Biochem Biophys Res Commun. 1979;90:1305–1312. doi: 10.1016/0006-291x(79)91178-1. [DOI] [PubMed] [Google Scholar]
- 11.Hasunuma Y, Matsuura E, Makita Z, Katahira T, Nishi S, Koike T. Involvement of beta 2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin Exp Immunol. 1997;107:569–573. doi: 10.1046/j.1365-2249.1997.d01-948.x. [DOI] [PubMed] [Google Scholar]
- 12.Hörkkö S, Miller E, Branch DW, Palinski W, Witztum JL. The epitopes for some antiphospholipid antibodies are adducts of oxidized phospholipid and beta2 glycoprotein 1 (and other proteins) Proc Natl Acad Sci USA. 1997;94:10356–10361. doi: 10.1073/pnas.94.19.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin KY, Pan JP, Yang DL, Huang KT, Chang MS, Ding PY, Chiang AN. Evidence for inhibition of low density lipoprotein oxidation and cholesterol accumulation by apolipoprotein H (beta2-glycoprotein I) Life Sci. 2001;69:707–719. doi: 10.1016/s0024-3205(01)01164-x. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen T, Schousboe I, Boel E, Mulvihill EM, Hansen RR, Moller KB, Moller NPH, Sottrup-Jensen L. Molecular cloning and mammalian expression of human β2-glycoprotein I cDNA. FEBS Lett. 1991;289:183–186. doi: 10.1016/0014-5793(91)81065-g. [DOI] [PubMed] [Google Scholar]
- 15.Lozier J, Takahashi N, Putnam FW. Complete amino acid sequence of human plasmid β2-glycoprotein I. Proc Natl Acad Sci USA. 1984;81:3640–3644. doi: 10.1073/pnas.81.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehdi H, Nunn M, Steel DM, Whitehead AS, Perez M, Walker L, Peeples ME. Nucleotide sequence and expression of the human gene encoding apolipoprotein H. Gene. 1991;108:293–298. doi: 10.1016/0378-1119(91)90449-l. [DOI] [PubMed] [Google Scholar]
- 17.Steinkasserer A, Estaller C, Weiss E, Sim RB. Complete nucleotide and deduced amino acid sequence of human β2-glycoprotein I. Biochem J. 1991;277:387–391. doi: 10.1042/bj2770387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato H, Enjyoji KI. Amino acid sequence and location of the disulfide bonds in bovine β2-glycoprotein I: The presence of five sushi domains. Biochemistry. 1991;30:1687–1694. doi: 10.1021/bi00114a012. [DOI] [PubMed] [Google Scholar]
- 19.Aoyama Y, Chan YL, Wool IG. The primary structure of rat β2glycoprotein I. Nucl Acid Res. 1989;17:6401. doi: 10.1093/nar/17.15.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nonaka M, Matsuda Y, Shirojshi T, Moriwaki K, Nonaka M, Natsuume-Sakai S. Molecular cloning of mouse β2-glycoprotein I and mapping of the gene to chrosome 11. Genomics. 1992;13:1082–1087. doi: 10.1016/0888-7543(92)90022-k. [DOI] [PubMed] [Google Scholar]
- 21.Sellar GC, Keane J, Mehdi H, Peeoles ME, Browne N, Whitehead AS. Characterization and acute phase modulation of canine apolipoprotein H (β2-glycoprotein I) Biochem Biophys Res Commun. 1993;191:1288–1293. doi: 10.1006/bbrc.1993.1357. [DOI] [PubMed] [Google Scholar]
- 22.Wurm H. β2-glycoprotein-I (apolipoprotein H) interactions with phospholipid vesicles. Int J Biochem. 1984;16:511–515. doi: 10.1016/0020-711x(84)90168-x. [DOI] [PubMed] [Google Scholar]
- 23.Jones JV, James H, Tan MH, Mansouor M. Antiphospholipid antibodies required β2-glycoprotein I (apolipoprotein H) as cofactor. J Rhematol. 1992;19:1397–1402. [PubMed] [Google Scholar]
- 24.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid binding inhibitor of coagulation: rβ2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanghera DK, Kristensen T, Hamman RF, Kamboh MI. Molecular basis of the apolipoprotein H (β2-glycoprotein I) protein polymorphism. Hum Genet. 1997;100:57–62. doi: 10.1007/s004390050465. [DOI] [PubMed] [Google Scholar]
- 26.Steinkasserer A, Dorner C, Wurzner R, Sim RB. Human β2-glycoprotein I: molecular analysis of DNA and amino acid polymorphism. Hum Genet. 1993;91:401–402. doi: 10.1007/BF00217367. [DOI] [PubMed] [Google Scholar]
- 27.Sanghera DK, Wagenknecht DR, Mcintyre JA, Kamboh MI. Identification of structural mutations in the fifth domain of apolipoprotein H (β2glycoprotein I) which affect phospholipid binding. Hum Mol Genet. 1997;6:311–316. doi: 10.1093/hmg/6.2.311. [DOI] [PubMed] [Google Scholar]
- 28.Mehdi H, Naqvi A, Kamboh MI. A hydrophobic sequence at position 313316 (Leu-Ala-Phe-Trp) in the fifth domain of apolipoprotein H (rβ2-glycoprotein I) is crucial for cardiolipin binding. Eur J Biochem. 2000;267:1770–1776. doi: 10.1046/j.1432-1327.2000.01174.x. [DOI] [PubMed] [Google Scholar]
- 29.Sanger F, Nicklin S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehdi H, Ono E, Gupta KC. Initiation of translation at CUG, GUG, and ACG codons in mammalian cells. Gene. 1990;91:173–178. doi: 10.1016/0378-1119(90)90085-6. [DOI] [PubMed] [Google Scholar]
- 31.Miyanohara A, Toh-e A, Nozaiki C, Hamada F, Ohtomo N, Matsubara K. Expression of hepatitis B surface antigen gene in yeast. Proc Natl Acad Sci USA. 1993;80:1–5. doi: 10.1073/pnas.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenzuela P, Medina A, Rutter WJ, Ammerer G, Hall BD. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298:347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- 33.Gao PJ, Piao YF, Liu XD, Qu LK, Shi Y, Wang XC, Tang HY. Studies on specific interaction of beta-2-glycoprotein I with HBsAg. World J Gastroenterol. 2003;9:2114–2116. doi: 10.3748/wjg.v9.i9.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao PJ, Shi Y, Gao YH, Liu YW, Tang Y. The receptor β2GPI on membrane of hepatocellular carcinoma cell line SMMC-7721 is annexin II. World J Gastroenterol. 2007;13:3364–3368. doi: 10.3748/wjg.v13.i24.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefas I, Rucheton M, D’Angeac AD, Morel-Baccard C, Seigneurin JM, Zarski JP, Martin M, Cerutti M, Bossy JP, Misse D, Graafland H, Veas F. Hepatitis B virus Dane particles bind to human plasma apolipoprotein H. Hepatology. 2001;33:207–217. doi: 10.1053/jhep.2001.20531. [DOI] [PubMed] [Google Scholar]
- 36.Neurath AR, Strick N. The putative cell receptors for hepatitis B virus (HBV), annexin V, and apolipoprotein H, bind to lipid components of HBV. Virology. 1994;204:475–477. doi: 10.1006/viro.1994.1558. [DOI] [PubMed] [Google Scholar]
- 37.Bouma B, de Groot PG, van den Elsen JMH, Ravelli RBG, Schouten A, Simmelink MJA, Derksen RHWM, Kroon J, Gras P. Adhesion mechanism of human β2-glycoprotein I to phospholipids based on its crystal structure. EMBO J. 1999;18:5166–5174. doi: 10.1093/emboj/18.19.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarzenbacher R, Zeth K, Diedericks K, Gries A, Kostner GM, Laggner P, Prassl R. Crystal structure of human β2-glycoprotein I: implications for phospholipid binding and the antiphospholipid syndrome. EMBO J. 1999;18:6228–6239. doi: 10.1093/emboj/18.22.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoshino M, Hagihara Y, Nishii I, Yamazaki T, Kato H, Goto Y. Identification of the phospholipid-binding site of human beta(2)-glycoprotein I domain V by heteronuclear magnetic resonance. J Mol Biol. 2000;304:927–939. doi: 10.1006/jmbi.2000.4243. [DOI] [PubMed] [Google Scholar]