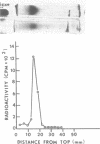
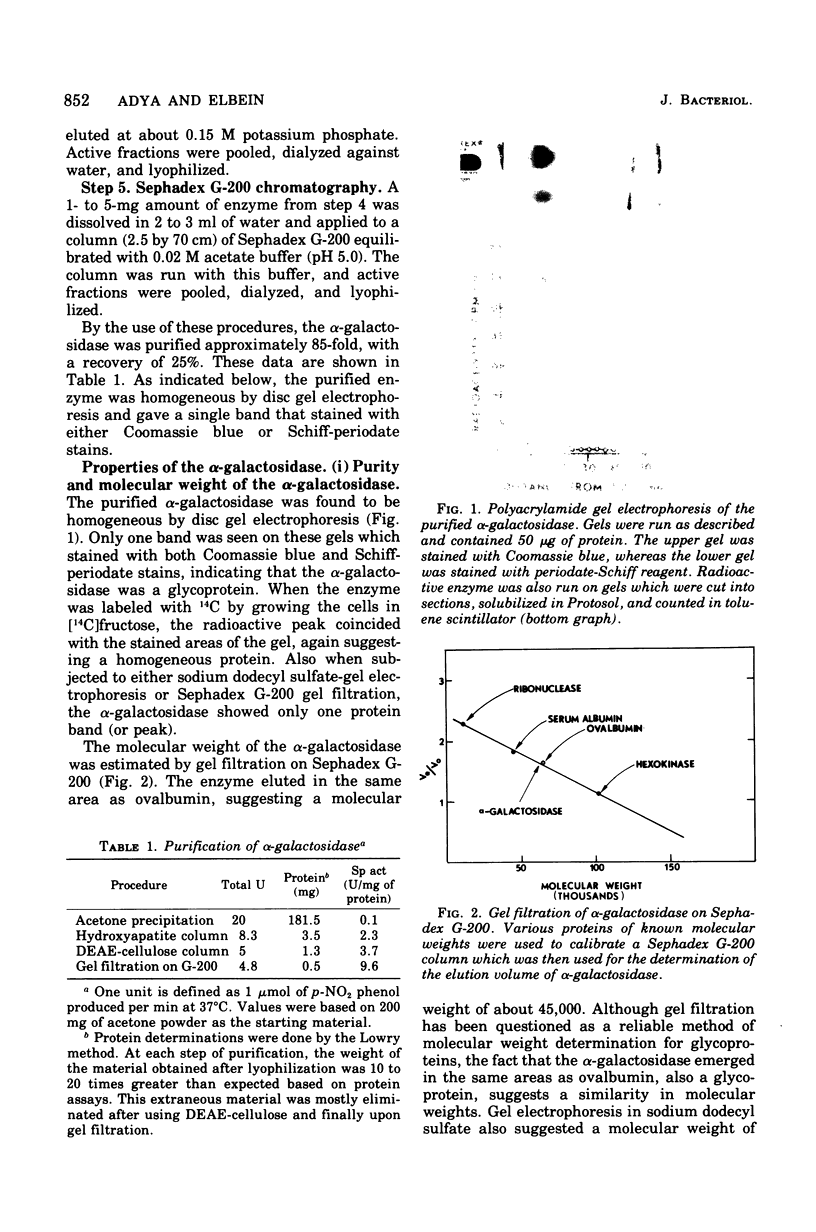
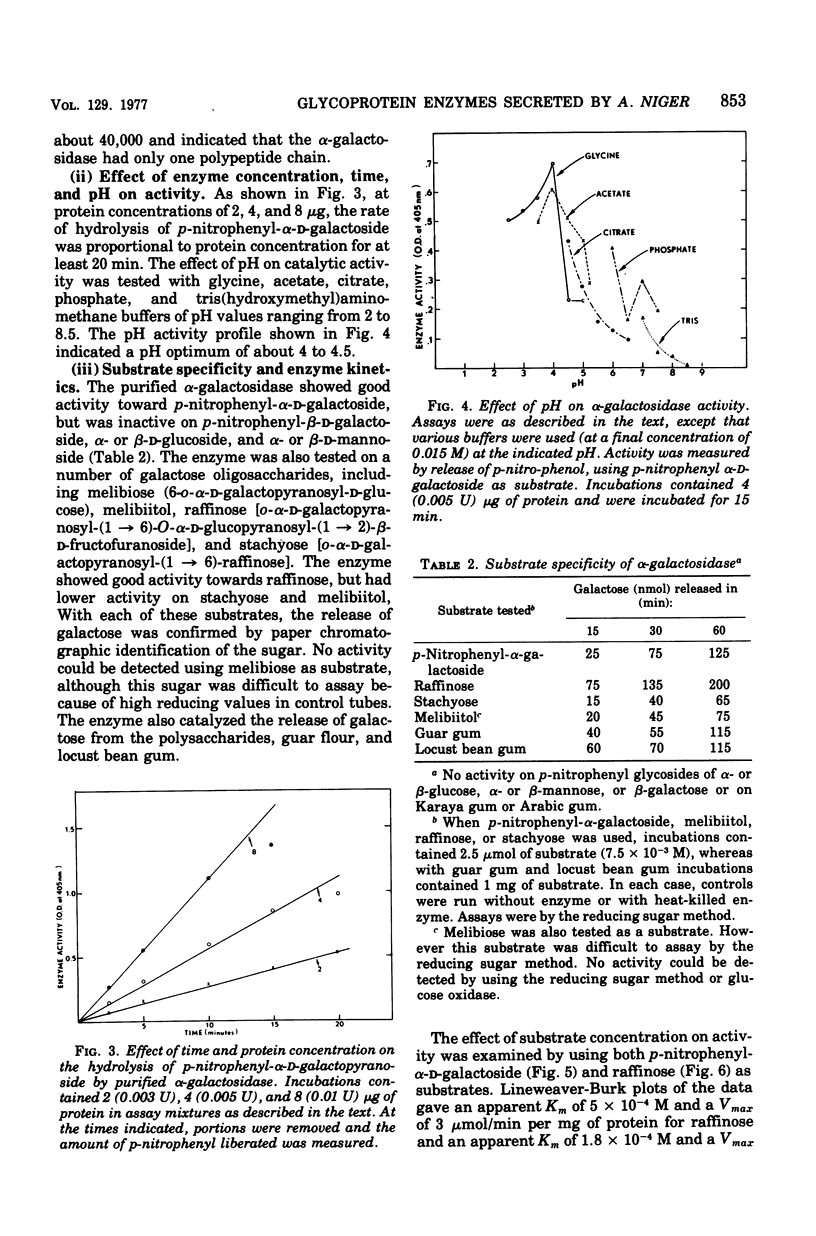
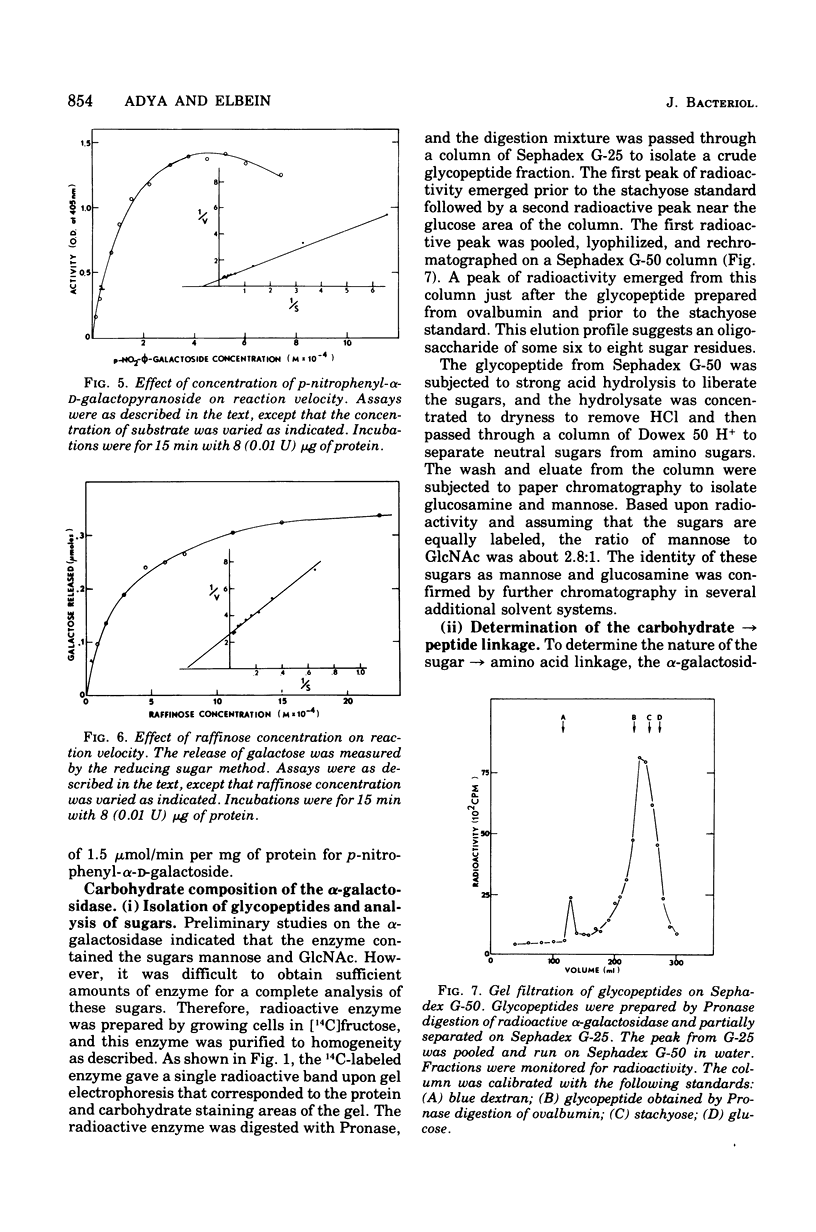
Abstract
An alpha-galactosidase (alpha-D-galactoside galactohydrolase [EC 3.2.1.22]) was purified to homogeneity from the culture filtrate of Aspergillus niger. The enzyme had an apparent molecular weight of 45,000 and was a glycoprotein. Radioactive enzyme was prepared by growing cells in [14C]fructose and this enzyme was used to prepare 14C-labeled glycopeptides. The glycopeptides emerged from Sephadex G-50 between stachyose and the glycopeptide from ovalbumin. Based on calibration of the column with various-sized dextran oligosaccharides, the glycopeptides appeared to have a molecular weight of 1,200 to 1,400. Analysis of the glycopeptide(s) indicated that it contained mannose and N-acetylglucosamine (GlcNAc) in an approximate ratio of 3 or 4 to 1. Assuming that there are two GlcNAc residues in the oligosaccharide and based on the molecular weight of the glycopeptide, the oligosaccharide probably contains eight to nine sugar residues. Alks probably attached to the protein by a GlcNAc leads to asparagine linkage. The purified alpha-galactosidase was most active on raffinose (Km = 5 x 10--4 M, Vmax = 3 mumol/min per mg of protein), but also showed good activity on p-nitrophenyl-alpha-D-galactoside ans somewhat less activity on stachyose and melibitol. The enzyme also hydrolyzed guar flour and locust bean gum, but did not attack the p-nitrophenyl glycosides of beta-galactose, alpha- or beta-glucose, or alpha- or beta-mannose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima T., Spiro R. G. Studies on the carbohydrate units of thyroglobulin. Structure of the mannose-N-acetylglucosamine unit (unit A) of the human and calf proteins. J Biol Chem. 1972 Mar 25;247(6):1836–1848. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Johnson A. R. Improved method of hexosamine determination. Anal Biochem. 1971 Dec;44(2):628–635. doi: 10.1016/0003-2697(71)90252-1. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. Y., Scocca J. R. A common structural unit in asparagine-oligosaccharides of several glycoproteins from different sources. J Biol Chem. 1972 Sep 25;247(18):5753–5758. [PubMed] [Google Scholar]
- Rudick M. J., Elbein A. D. Glycoprotein enzymes secreted by Aspergillus fumigatus. Purification and properties of beta-glucosidase. J Biol Chem. 1973 Sep 25;248(18):6506–6513. [PubMed] [Google Scholar]
- Rudick M. J., Elbein A. D. Glycoprotein enzymes secreted by Aspergillus fumigatus: purification and properties of a second beta-glucosidase. J Bacteriol. 1975 Oct;124(1):534–541. doi: 10.1128/jb.124.1.534-541.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. A re-evaluation of the oligosaccharide sequence associated with ovalbumin. J Biol Chem. 1972 Apr 25;247(8):2629–2631. [PubMed] [Google Scholar]
- Tarentino A., Plummer T. H., Jr, Maley F. Studies on the oligosaccharide sequence of ribonuclease B. J Biol Chem. 1970 Aug 25;245(16):4150–4157. [PubMed] [Google Scholar]
- Yamaguchi H., Ikenaka T., Matsushima Y. The complete sequence of a glycopeptide obtained from Taka-amylase A. J Biochem. 1971 Oct;70(4):587–594. doi: 10.1093/oxfordjournals.jbchem.a129675. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]