Abstract
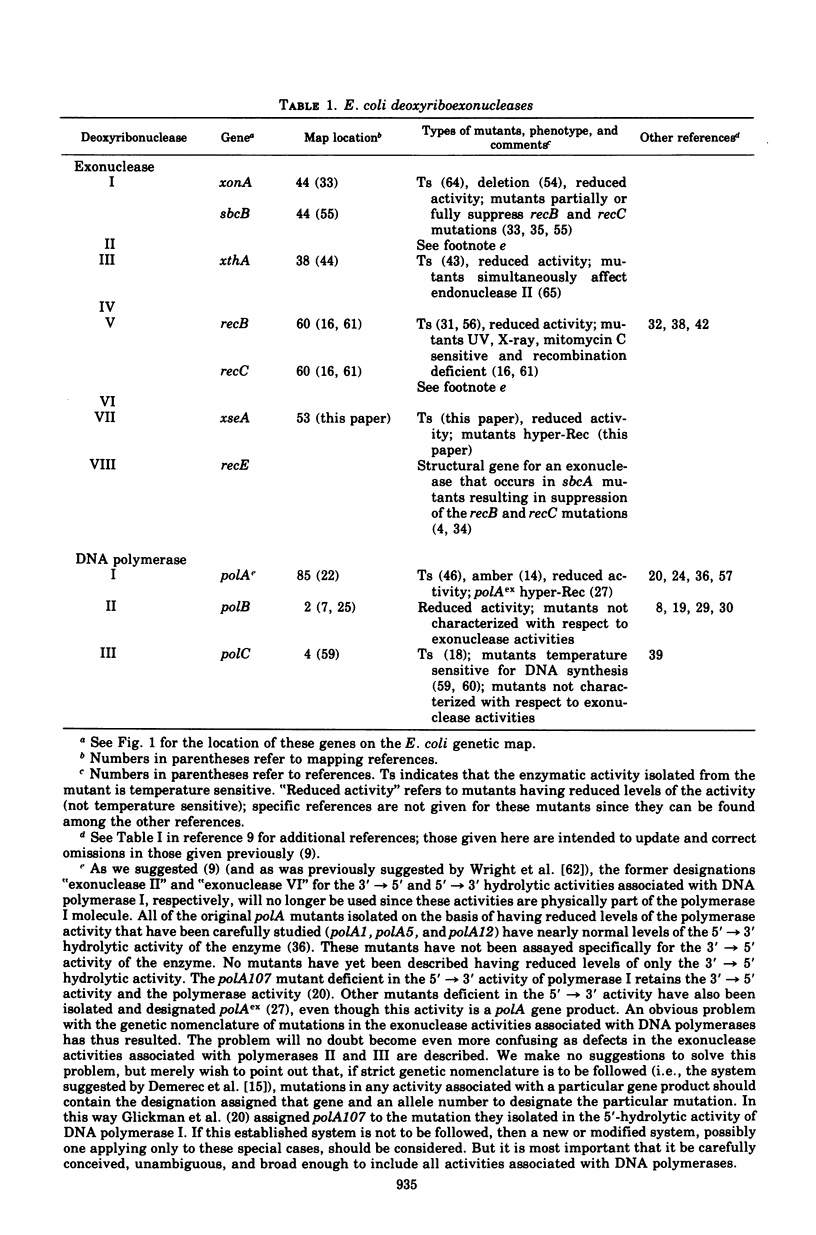
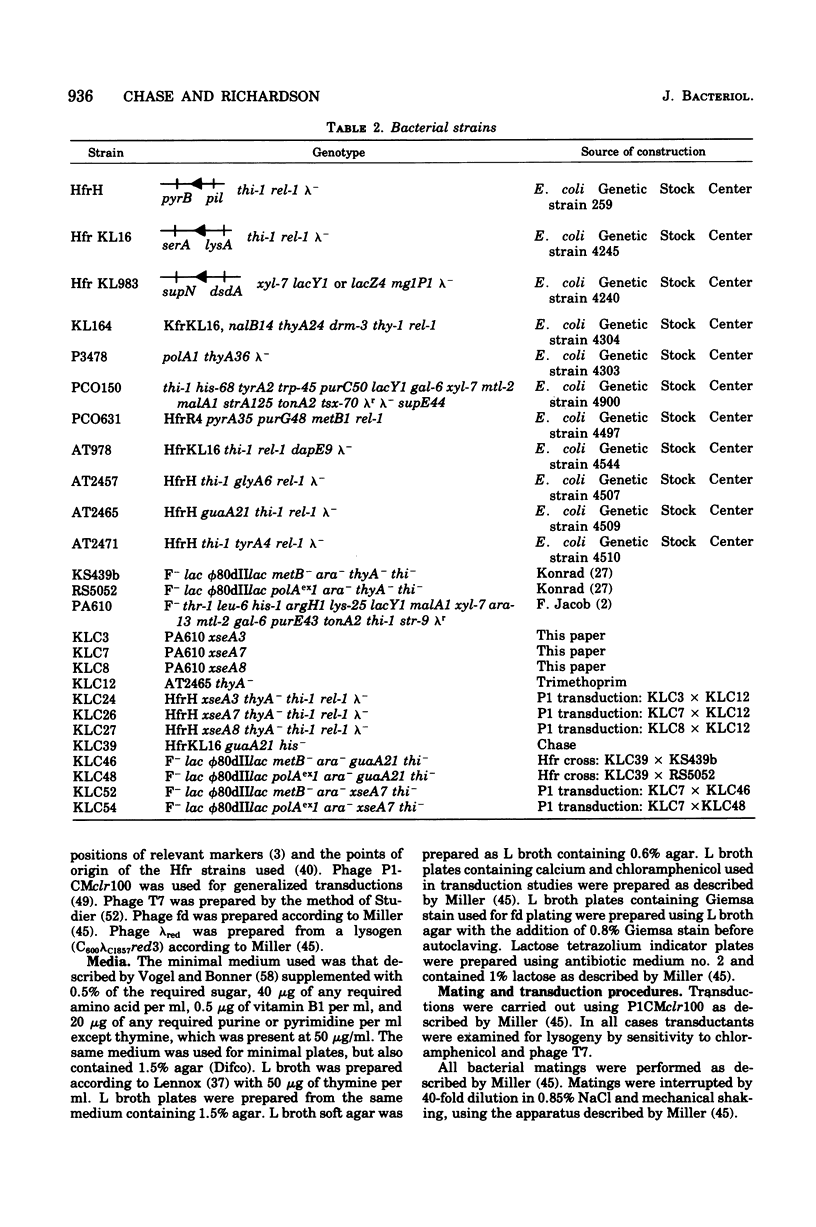
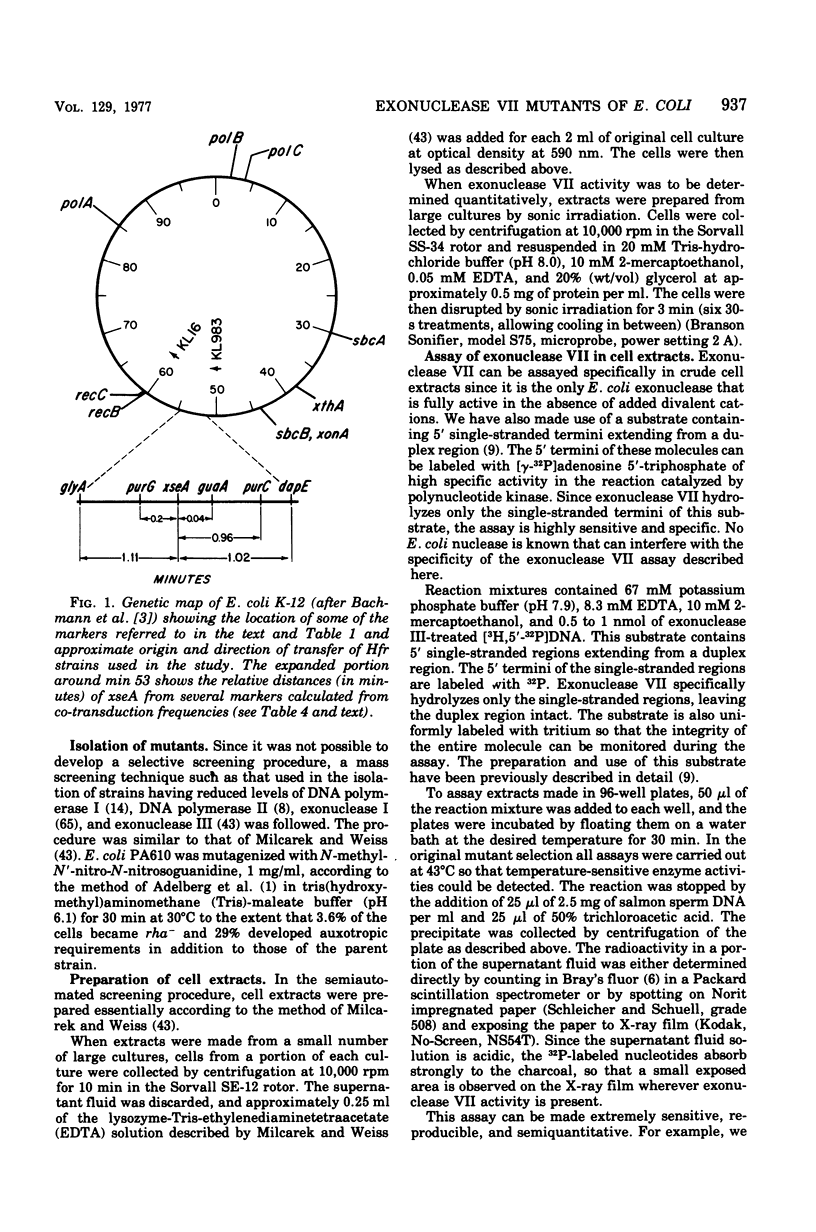
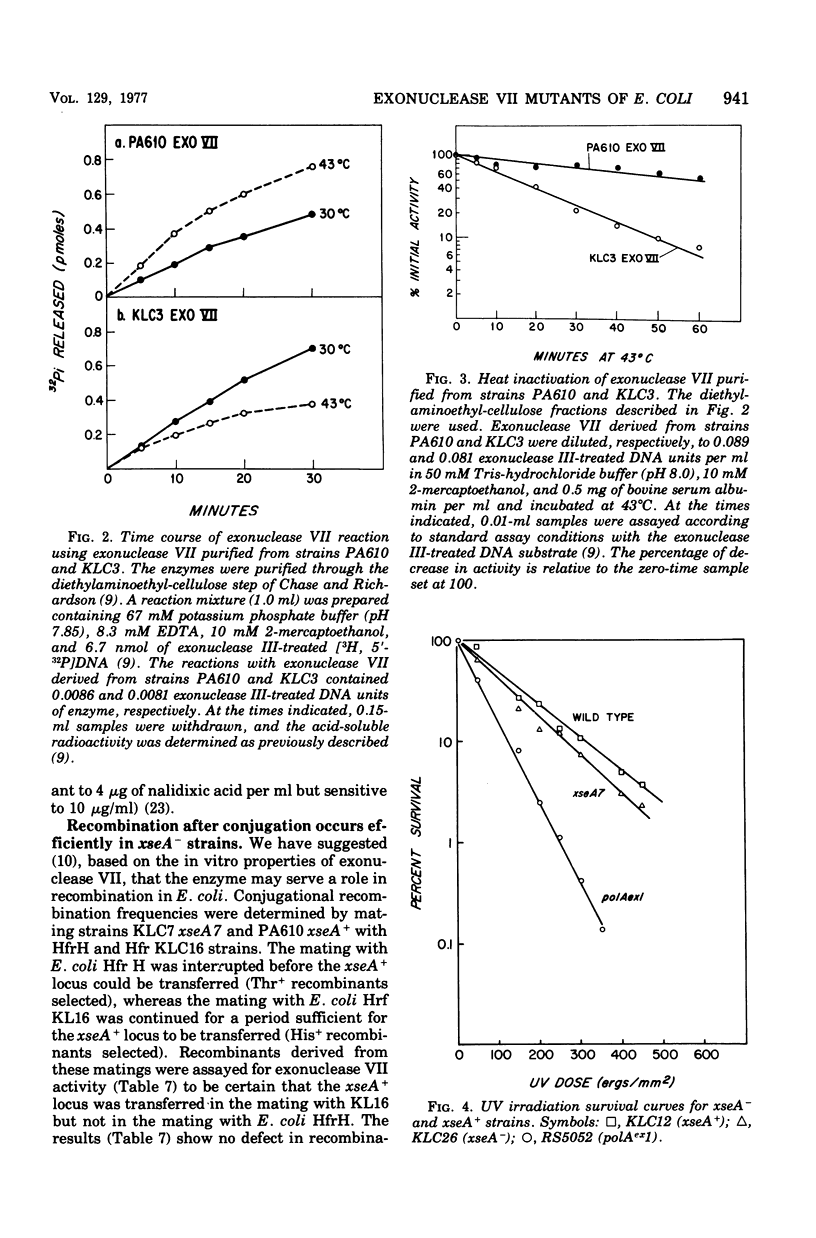
Mutants of Escherichia coli having reduced levels of exonuclease VII activity have been isolated by a mass screening procedure. Nine mutants, five of which are known to be of independent origin, were obtained and designated xse. The defects in these strains lie at two or more loci. One of these loci, xseA, lies in the interval between purG and purC; it is 93 to 97% co-transducible with guaA. The order of the genes in this region is purG-xseA guaA,B-purC. The available data do not allow xseA to be ordered with respect to guaA,B. Exonuclease VII purified from E. coli KLC3 xseA3 is more heat labile than exonuclease VII purified from the parent, E. coli PA610 xse+. Therefore, xseA is the structural gene for exonuclease VII. Mutants with defects in the xseA gene show increased sensitivity to nalidixic acid and have an abnormally high frequency of recombination (hyper-Rec phenotype) as measured by the procedure of Konrad and Lehlman (1974). The hyper-Rec character of xseA strains is approximately one-half that of the polAex1 mutant defective in the 5' leads to 3' hydrolytic activity of deoxyribonucleic acid polymerase I. The double mutant, polAex1 xseA7, is twice as hyper-Rec as the polAex1 mutant alone. The xseA- strains are slightly more sensitive to ultraviolet irradiation than the parent strain. Bacteriophages T7, fd, and lambdared grow normally in xseA- strains.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon G. J., Levitt M., Sternglanz R. Studies on the mechanism of action of nalidixic acid. Antimicrob Agents Chemother. 1973 Oct;4(4):479–486. doi: 10.1128/aac.4.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Shizuya H., Richardson C. C. Mapping of a mutation, polB100, affecting deoxyribonucleic acid polymerase II in Escherichia coli K-12. J Bacteriol. 1974 Aug;119(2):494–499. doi: 10.1128/jb.119.2.494-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Mechanism of action. J Biol Chem. 1974 Jul 25;249(14):4553–4561. [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Toward a metabolic interpretation of genetic recombination of E. coli and its phages. Annu Rev Microbiol. 1971;25:437–464. doi: 10.1146/annurev.mi.25.100171.002253. [DOI] [PubMed] [Google Scholar]
- Cook T. M., Brown K. G., Boyle J. V., Goss W. A. Bactericidal action of nalidixic acid on Bacillus subtilis. J Bacteriol. 1966 Nov;92(5):1510–1514. doi: 10.1128/jb.92.5.1510-1514.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E. A., Smith H. O. Production of possible recombination intermediates by an ATP-dependent DNAase. Nat New Biol. 1973 Jan 10;241(106):54–58. doi: 10.1038/newbio241054a0. [DOI] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman B. W., van Sluis C. A., Heijneker H. L., Rörsch A. A mutant of Escherichia coli K12 deficient in the 5'-3' exonucleolytic activity of DNA polymerase I. I. General characterization. Mol Gen Genet. 1973 Jul 31;124(1):69–82. doi: 10.1007/BF00267166. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijneker H. L., Ellens D. J., Tjeerde R. H., Glickman B. W., van Dorp B., Pouwels P. H. A mutant of Escherichia coli K12 deficient in the 5'-3' exonucleolytic activity of DNA polymerase I. II. Purification and properties of the mutant enzyme. Mol Gen Genet. 1973 Jul 31;124(1):83–96. doi: 10.1007/BF00267167. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Gefter M., Mindich L. A mutant of Escherichia coli defective in DNA polymerase II activity. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3238–3242. doi: 10.1073/pnas.69.11.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. A conditional lethal mutant of Escherichia coli K12 defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. Proc Natl Acad Sci U S A. 1974 May;71(5):2048–2051. doi: 10.1073/pnas.71.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. Novel mutants of Escherichia coli that accumulate very small DNA replicative intermediates. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2150–2154. doi: 10.1073/pnas.72.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. DNA synthesis in cell-free extracts of a DNA polymerase-defective mutant. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1348–1355. doi: 10.1016/0006-291x(70)90014-8. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. Purification and DNA synthesis in cell-free extracts: properties of DNA polymerase II. Proc Natl Acad Sci U S A. 1971 Apr;68(4):761–764. doi: 10.1073/pnas.68.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R. Differential thermolability of exonuclease and endonuclease activities of the recBC nuclease isolated from thermosensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1219–1222. doi: 10.1128/jb.120.3.1219-1222.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. doi: 10.1128/jb.120.3.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Clark A. J. Isolation of exonuclease VIII: the enzyme associated with sbcA indirect suppressor. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3593–3597. doi: 10.1073/pnas.71.9.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman I. R., Chien J. R. Persistence of deoxyribonucleic acid polymerase I and its 5'--3' exonuclease activity in PolA mutants of Escherichia coli K12. J Biol Chem. 1973 Nov 25;248(22):7717–7723. [PubMed] [Google Scholar]
- Lieberman R. P., Oishi M. The recBC deoxyribonuclease of Escherichia coli: isolation and characterization of the subunit proteins and reconstitution of the enzyme. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4816–4820. doi: 10.1073/pnas.71.12.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Characterization of associated exonuclease activities. J Biol Chem. 1975 Jan 25;250(2):470–478. [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay V., Linn S. The mechanism of degradation of duplex deoxyribonucleic acid by the recBC enzyme of Escherichia coli K-12. J Biol Chem. 1974 Jul 10;249(13):4286–4294. [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. I. Isolation and characterization of mutants for exonuclease 3. J Mol Biol. 1972 Jul 21;68(2):303–318. doi: 10.1016/0022-2836(72)90215-x. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Preliminary mapping of mutations affecting exonuclease 3 in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):1086–1088. doi: 10.1128/jb.113.2.1086-1088.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijkamp H. J., De Haan P. G. Genetic and biochemical studies of the guanosine 5'-monophosphate pathway in Escherichia coli. Biochim Biophys Acta. 1967 Aug 22;145(1):31–40. doi: 10.1016/0005-2787(67)90651-x. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., Oskamp A. A. Regulation of the biosynthesis of guanosine 5'-monophosphate: evidence for one operon. J Mol Biol. 1968 Jul 14;35(1):103–109. doi: 10.1016/s0022-2836(68)80040-3. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Storm P. K., Hoekstra W. P., de Haan P. G., Verhoef C. Genetic recombination in Escherichia coli. IV. Isolation and characterization of recombination-deficiency mutants of Escherichia coli K12. Mutat Res. 1971 Sep;13(1):9–17. doi: 10.1016/0027-5107(71)90121-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Yudelevich A., Hurwitz J. DNA synthesis in vitro dependent upon phiX174 replicative form I DNA. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1887–1891. doi: 10.1073/pnas.73.6.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine M. G., Kelly B. Extent of host deletions associated with bacteriophage P2-mediated eduction. J Bacteriol. 1971 Nov;108(2):695–704. doi: 10.1128/jb.108.2.695-704.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin A., Kushner S. R., Clark A. J. Genetic analysis of mutations indirectly suppressing recB and recC mutations. Genetics. 1972 Oct;72(2):105–115. [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Uyemura D., Eichler D. C., Lehman I. R. Biochemical characterization of mutant forms of DNA polymerase I from Escherichia coli. II. The polAex1 mutation. J Biol Chem. 1976 Jul 10;251(13):4085–4089. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Nüsslein V., Otto B., Klein A., Bonhoeffer F., Herrmann R., Gloger L., Schaller H. Isolation and characterization of thermosensitive Escherichia coli mutants defective in deoxyribonucleic acid replication. J Bacteriol. 1973 Mar;113(3):1381–1388. doi: 10.1128/jb.113.3.1381-1388.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Valentine M. C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. II. Isolation and characterization of mutants for exonuclease I. J Mol Biol. 1974 May 15;85(2):323–343. doi: 10.1016/0022-2836(74)90367-2. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Weiss B. Mutations simultaneously affecting endonuclease II and exonuclease III in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Feb;72(2):688–692. doi: 10.1073/pnas.72.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]



