Abstract
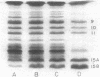
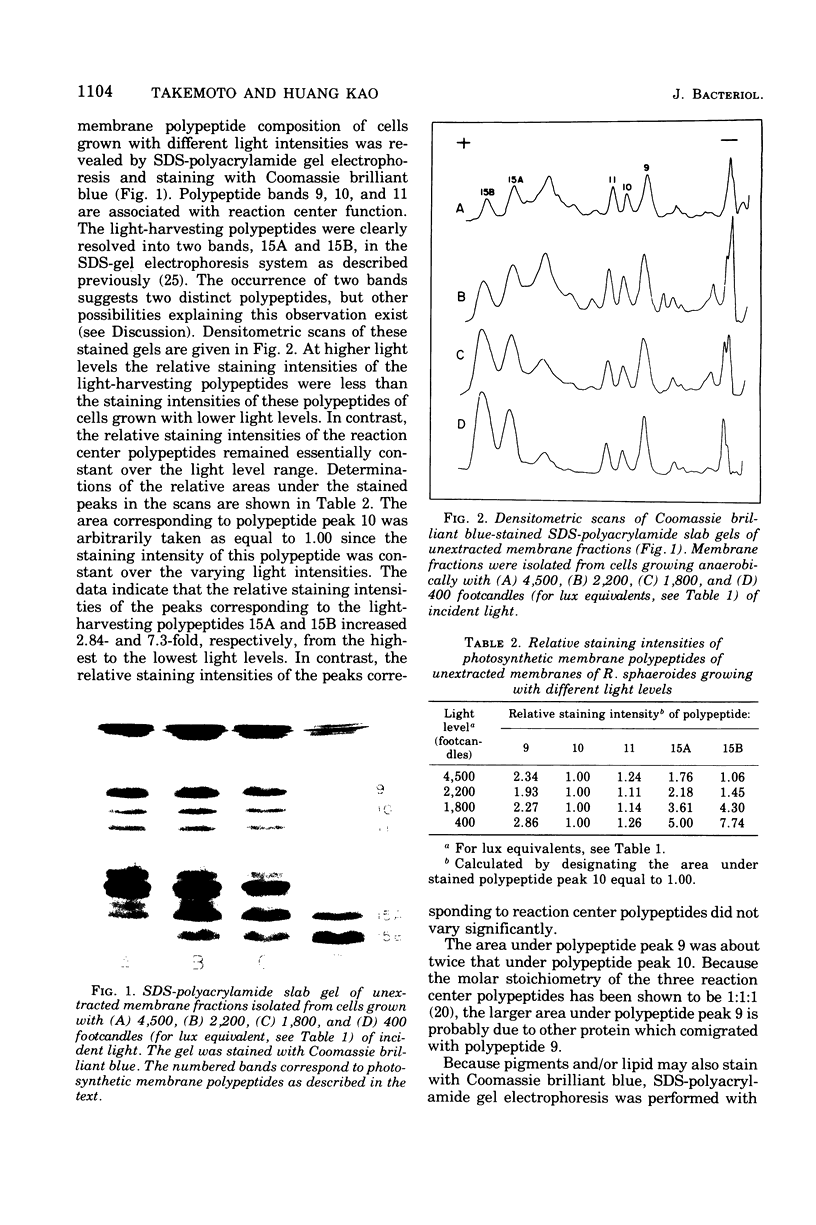
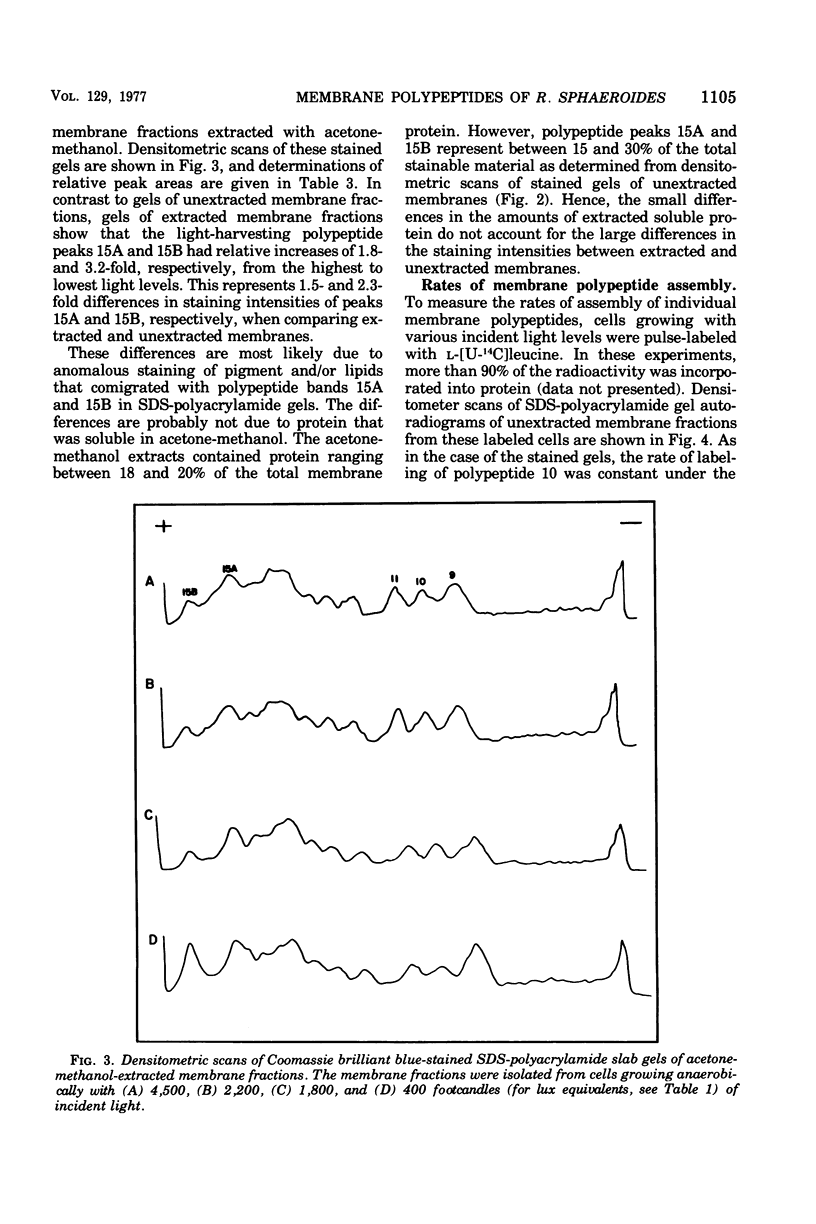
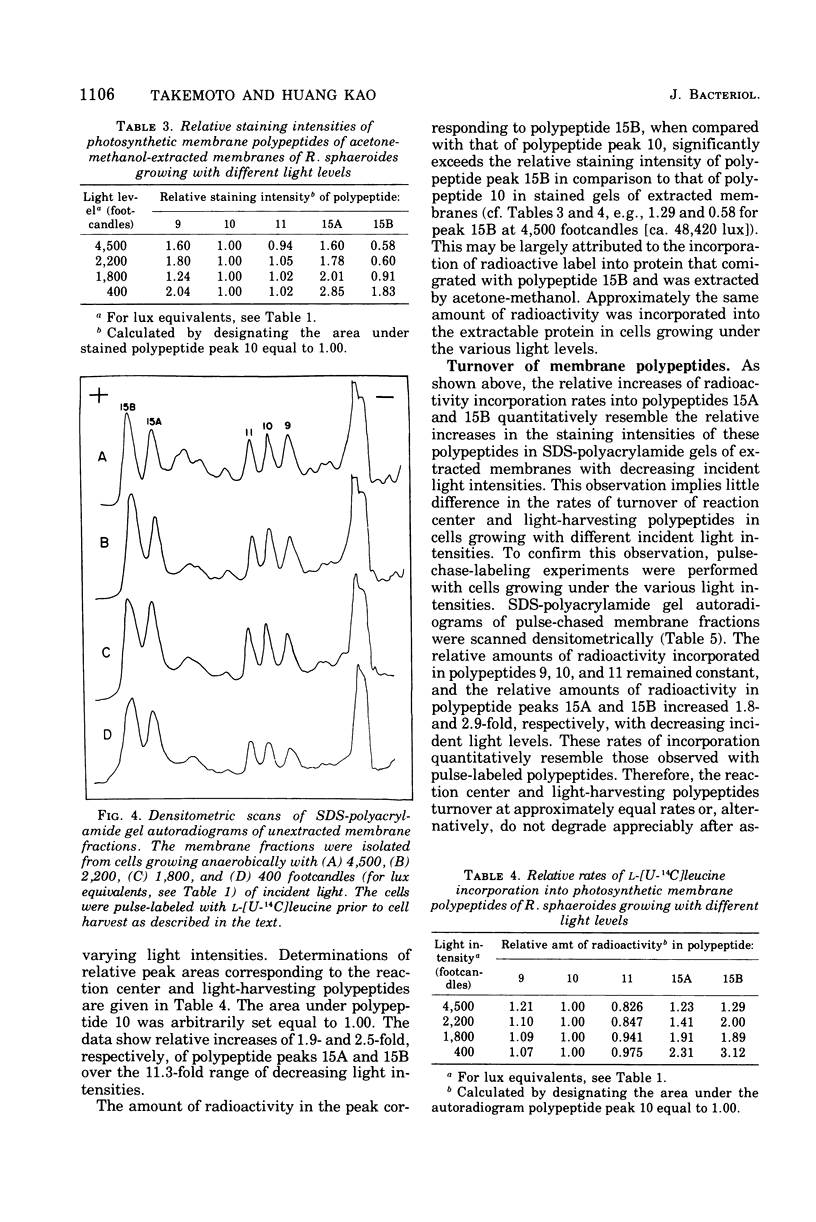
Cells of Rhodopseudomonas sphaeroides were grown anaerobically with incident light levels ranging between 4,500 and 400 footcandles (ca. 48,420 and 4,304 lux). Cells grown with the higher light levels had lower contents of total bacteriochlorophyll and incorporated L-[U-14C]leucine into membrane protein at higher rates than cells grown with lower light levels. The former cells also contained relatively lower amounts of light-harvesting membrane polypeptides as compared with the latter cells. In contrast, the relative amounts of reaction center membrane polypeptides were approximately the same with varying incident light levels. The relative amounts of these membrane polypeptides were correlated with differences in rates of synthesis and assembly of the polypeptides into membrane by measuring the rates of incorporation of L-[U-14C]leucine into the membrane-bound polypeptides. No significant differences in rates of turnover of these polypeptides were detected under the varying incident light levels as measured in pulse-chase radioactive labeling experiments.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard J., Sistrom W. R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972 Feb;15(2):209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Clayton B. J. Relations between pigments and proteins in the photosynthetic membranes of Rhodopseudomonas spheroides. Biochim Biophys Acta. 1972 Dec 14;283(3):492–504. doi: 10.1016/0005-2728(72)90265-4. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Haselkorn R. Protein components of bacterial photosynthetic membranes. J Mol Biol. 1972 Jul 14;68(1):97–105. doi: 10.1016/0022-2836(72)90265-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Feher G. Some chemical and physical properties of a bacterial reaction center particle and its primary photochemical reactants. Photochem Photobiol. 1971 Sep;14(3):373–387. doi: 10.1111/j.1751-1097.1971.tb06180.x. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Kaplan S. Isolation and characterization of a bacteriochlorophyll-containing protein from Rhodopseudomonas spheroides. J Biol Chem. 1972 May 10;247(9):2732–2737. [PubMed] [Google Scholar]
- Huang J. W., Kaplan S. Membrane proteins of Rhodopseudomonas spheroides. IV. Characterization of chromatophore proteins. Biochim Biophys Acta. 1973 May 11;307(2):317–331. doi: 10.1016/0005-2736(73)90098-9. [DOI] [PubMed] [Google Scholar]
- Irschik H., Oelze J. Membrane differentiation in phototrophically growing Rhodospirillum rubrum during transition from low to high light intensity. Biochim Biophys Acta. 1973 Nov 30;330(1):80–89. doi: 10.1016/0005-2736(73)90286-1. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Niederman R. A., Mallon D. E., Langan J. J. Membranes of Rhodopseudomonas sphaeroides. IV. Assembly of chromatophores in low-aeration cell suspensions. Biochim Biophys Acta. 1976 Aug 13;440(2):429–447. doi: 10.1016/0005-2728(76)90076-1. [DOI] [PubMed] [Google Scholar]
- Nieth K. F., Drews G. Formation of reaction centers and light-harvesting bacteriochlorophyll-protein complexes in Rhodopseudomonas capsulata. Arch Microbiol. 1975 Jun 20;104(1):77–82. doi: 10.1007/BF00447303. [DOI] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Oelze J., Pahike W. The early formation of the photosynthetic apparatus in Rhodospirillum rubrum. Arch Microbiol. 1976 Jul;108(3):281–285. doi: 10.1007/BF00454853. [DOI] [PubMed] [Google Scholar]
- Oelze J., Schroeder J., Drews G. Bacteriochlorophyll, fatty-acid, and protein synthesis in relation to thylakoid formation in mutant strains of Rhodospirillum rubrum. J Bacteriol. 1970 Mar;101(3):669–674. doi: 10.1128/jb.101.3.669-674.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M. Y., Steiner L. A., Feher G. Characterization of reaction centers from photosynthetic bacteria. I. Subunit structure of the protein mediating the primary photochemistry in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1394–1403. doi: 10.1021/bi00704a013. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R., CLAYTON R. K. STUDIES ON A MUTANT OF RHODOPSEUDOMONAS SPHEROIDES UNABLE TO GROW PHOTOSYNTHETICALLY. Biochim Biophys Acta. 1964 Jul 29;88:61–73. doi: 10.1016/0926-6577(64)90154-8. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. Observations on the relationship between the formation of photopigments and the synthesis of protein in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:599–605. doi: 10.1099/00221287-28-4-599. [DOI] [PubMed] [Google Scholar]
- Takemoto J. Kinetics of photosynthetic membrane protein assembly in Rhodopseudomonas spheroides. Arch Biochem Biophys. 1974 Aug;163(2):515–520. doi: 10.1016/0003-9861(74)90509-8. [DOI] [PubMed] [Google Scholar]
- Takemoto J., Lascelles J. Coupling between bacteriochlorophyll and membrane protein synthesis in Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):799–803. doi: 10.1073/pnas.70.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto J., Lascelles J. Function of membrane proteins coupled to bacteriochlorophyll synthesis. Studies with wild type and mutant strains of Rhodopseudomonas spheroides. Arch Biochem Biophys. 1974 Aug;163(2):507–514. doi: 10.1016/0003-9861(74)90508-6. [DOI] [PubMed] [Google Scholar]