Abstract
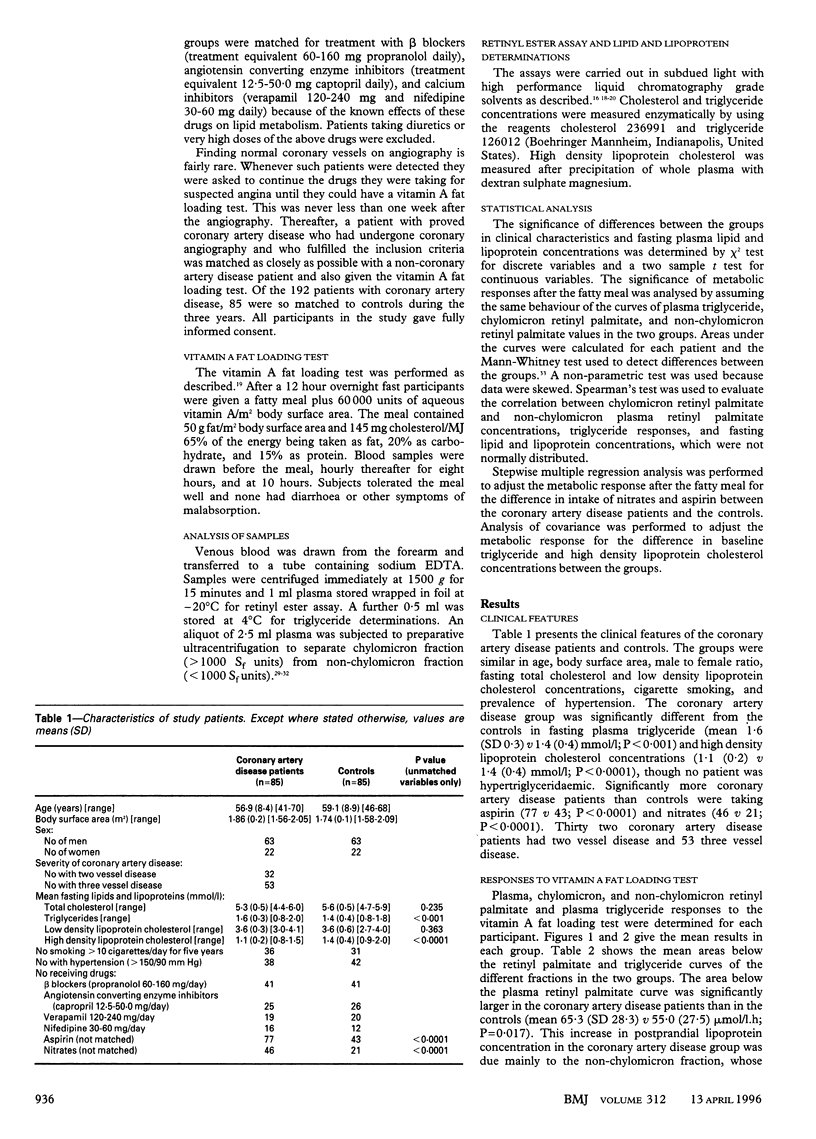
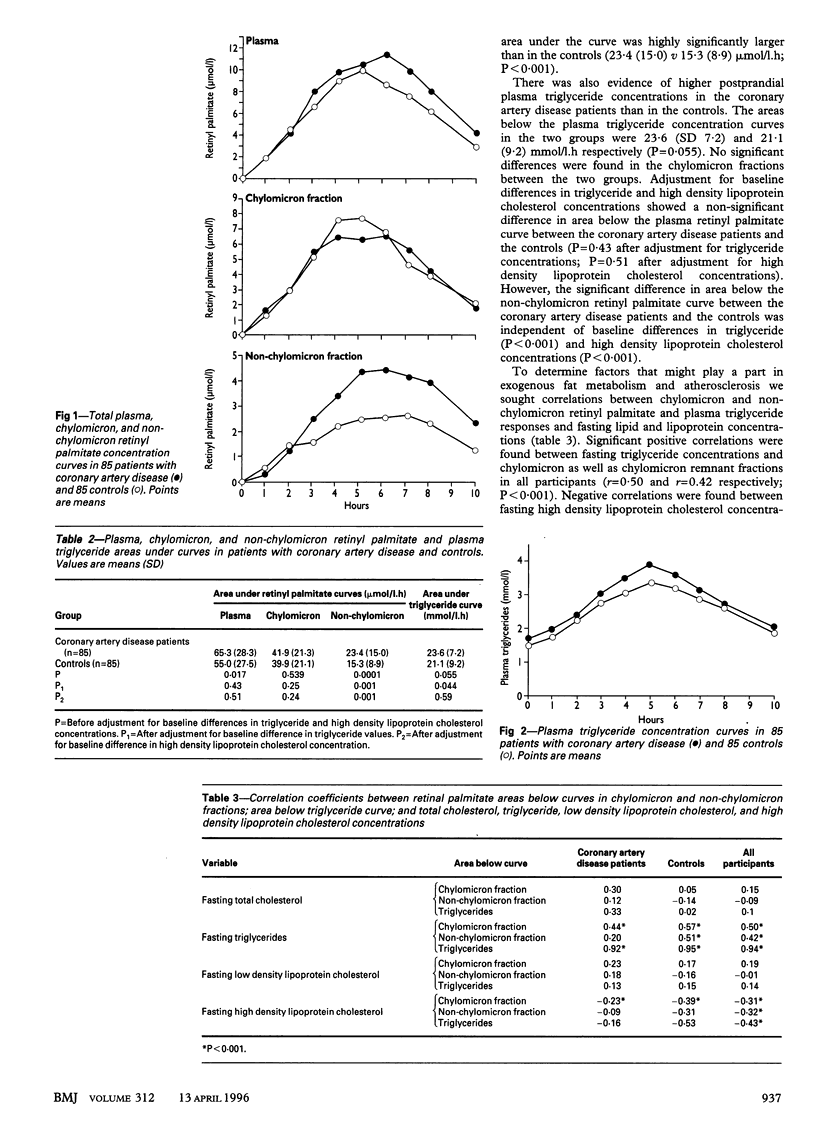
OBJECTIVE--To test the hypothesis that subjects who clear chylomicron remnants slowly from plasma may be at higher risk of coronary artery disease than indicated by their fasting plasma lipid concentrations. DESIGN--Case control study over three years. SETTING--An 800 bed general municipal hospital. SUBJECTS--85 normolipidaemic patients with coronary artery disease selected prospectively and matched with 85 normolipidaemic subjects with normal coronary arteries on angiography. INTERVENTIONS--All subjects were given a vitamin A fat loading test which specifically labels intestinal lipoproteins with retinyl palmitate. MAIN OUTCOME MEASURE--Postprandial lipoprotein metabolism. RESULTS--The area below the chylomicron remnant retinyl palmitate curve was significantly increased in the coronary artery disease group as compared with the controls (mean 23.4 (SD 15.0) v 15.3 (8.9) mumol/l.h; 95% confidence interval of difference 4.37 to 11.82). CONCLUSION--Normolipidaemic patients with coronary artery disease had significantly higher concentrations of chylomicron remnants in plasma than normolipidaemic subjects with normal coronary vessels. This may explain the mechanism underlying the susceptibility to atherosclerosis of coronary artery disease patients with normal fasting lipid values. As diet and drugs can ameliorate the accumulation of postprandial lipoproteins in plasma, the concentration of chylomicron remnants should be measured in patients at high risk of coronary artery disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berr F., Eckel R. H., Kern F., Jr Contraceptive steroids increase hepatic uptake of chylomicron remnants in healthy young women. J Lipid Res. 1986 Jun;27(6):645–651. [PubMed] [Google Scholar]
- Berr F., Eckel R., Kern F., Jr Plasma decay of chylomicron remnants is not affected by heparin-stimulated plasma lipolytic activity in normal fasting man. J Lipid Res. 1985 Jul;26(7):852–859. [PubMed] [Google Scholar]
- Berr F., Kern F., Jr Plasma clearance of chylomicrons labeled with retinyl palmitate in healthy human subjects. J Lipid Res. 1984 Aug;25(8):805–812. [PubMed] [Google Scholar]
- Blanchette-Mackie E. J., Scow R. O. Sites of lipoprotein lipase activity in adipose tissue perfused with chylomicrons. Electron microscope cytochemical study. J Cell Biol. 1971 Oct;51(1):1–25. doi: 10.1083/jcb.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortner J. A., Coates P. M., Le N. A., Cryer D. R., Ragni M. C., Faulkner A., Langer T. Kinetics of chylomicron remnant clearance in normal and in hyperlipoproteinemic subjects. J Lipid Res. 1987 Feb;28(2):195–206. [PubMed] [Google Scholar]
- DOLE V. P., HAMLIN J. T., 3rd Particulate fat in lymph and blood. Physiol Rev. 1962 Oct;42:674–701. doi: 10.1152/physrev.1962.42.4.674. [DOI] [PubMed] [Google Scholar]
- Demacker P. N., Reijnen I. G., Katan M. B., Stuyt P. M., Stalenhoef A. F. Increased removal of remnants of triglyceride-rich lipoproteins on a diet rich in polyunsaturated fatty acids. Eur J Clin Invest. 1991 Apr;21(2):197–203. doi: 10.1111/j.1365-2362.1991.tb01809.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984 Oct;25(10):1017–1058. [PubMed] [Google Scholar]
- Florén C. H., Albers J. J., Bierman E. L. Uptake of chylomicron remnants causes cholesterol accumulation in cultured human arterial smooth muscle cells. Biochim Biophys Acta. 1981 Jan 26;663(1):336–349. doi: 10.1016/0005-2760(81)90219-8. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S. The metabolism of chylomicron cholesterol ester in the rat. J Clin Invest. 1962 Oct;41:1886–1896. doi: 10.1172/JCI104645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianturco S. H., Lin A. H., Hwang S. L., Young J., Brown S. A., Via D. P., Bradley W. A. Distinct murine macrophage receptor pathway for human triglyceride-rich lipoproteins. J Clin Invest. 1988 Nov;82(5):1633–1643. doi: 10.1172/JCI113775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Brown M. S., Innerarity T. L., Mahley R. W. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteins. J Biol Chem. 1980 Mar 10;255(5):1839–1848. [PubMed] [Google Scholar]
- Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977 May;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- Groot P. H., van Stiphout W. A., Krauss X. H., Jansen H., van Tol A., van Ramshorst E., Chin-On S., Hofman A., Cresswell S. R., Havekes L. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb. 1991 May-Jun;11(3):653–662. doi: 10.1161/01.atv.11.3.653. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Mok H. Y. Chylomicron clearance in normal and hyperlipidemic man. Metabolism. 1976 Nov;25(11):1225–1239. doi: 10.1016/s0026-0495(76)80006-6. [DOI] [PubMed] [Google Scholar]
- Hazzard W. R., Bierman E. L. Delayed clearance of chylomicron remnants following vitamin-A-containing oral fat loads in broad-beta disease (type III hyperlipoproteinemia). Metabolism. 1976 Jul;25(7):777–801. doi: 10.1016/0026-0495(76)90149-9. [DOI] [PubMed] [Google Scholar]
- Hollander D. Intestinal absorption of vitamins A, E, D, and K. J Lab Clin Med. 1981 Apr;97(4):449–462. [PubMed] [Google Scholar]
- Hui D. Y., Brecht W. J., Hall E. A., Friedman G., Innerarity T. L., Mahley R. W. Isolation and characterization of the apolipoprotein E receptor from canine and human liver. J Biol Chem. 1986 Mar 25;261(9):4256–4267. [PubMed] [Google Scholar]
- Hui D. Y., Innerarity T. L., Mahley R. W. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981 Jun 10;256(11):5646–5655. [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem. 1955 Jul;215(1):1–14. [PubMed] [Google Scholar]
- Kane J. P., Chen G. C., Hamilton R. L., Hardman D. A., Malloy M. J., Havel R. J. Remnants of lipoproteins of intestinal and hepatic origin in familial dysbetalipoproteinemia. Arteriosclerosis. 1983 Jan-Feb;3(1):47–56. doi: 10.1161/01.atv.3.1.47. [DOI] [PubMed] [Google Scholar]
- Karpe F., Bell M., Björkegren J., Hamsten A. Quantification of postprandial triglyceride-rich lipoproteins in healthy men by retinyl ester labeling and simultaneous measurement of apolipoproteins B-48 and B-100. Arterioscler Thromb Vasc Biol. 1995 Feb;15(2):199–207. doi: 10.1161/01.atv.15.2.199. [DOI] [PubMed] [Google Scholar]
- Krasinski S. D., Cohn J. S., Russell R. M., Schaefer E. J. Postprandial plasma vitamin A metabolism in humans: a reassessment of the use of plasma retinyl esters as markers for intestinally derived chylomicrons and their remnants. Metabolism. 1990 Apr;39(4):357–365. doi: 10.1016/0026-0495(90)90249-c. [DOI] [PubMed] [Google Scholar]
- Lippel K., Tyroler H., Eder H., Gotto A., Jr, Vahouny G. Relationship of hypertriglyceridemia to atherosclerosis. Arteriosclerosis. 1981 Nov-Dec;1(6):406–417. doi: 10.1161/01.atv.1.6.406. [DOI] [PubMed] [Google Scholar]
- Matthews J. N., Altman D. G., Campbell M. J., Royston P. Analysis of serial measurements in medical research. BMJ. 1990 Jan 27;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. E., Hammett F., Saltissi S., Rao S., van Zeller H., Coltart J., Lewis B. Relation of angiographically defined coronary artery disease to plasma lipoprotein subfractions and apolipoproteins. Br Med J (Clin Res Ed) 1981 May 30;282(6278):1741–1744. doi: 10.1136/bmj.282.6278.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganroth J., Levy R. I., Fredrickson D. S. The biochemical, clinical, and genetic features of type III hyperlipoproteinemia. Ann Intern Med. 1975 Feb;82(2):158–174. doi: 10.7326/0003-4819-82-2-158. [DOI] [PubMed] [Google Scholar]
- Patsch J. R., Karlin J. B., Scott L. W., Smith L. C., Gotto A. M., Jr Inverse relationship between blood levels of high density lipoprotein subfraction 2 and magnitude of postprandial lipemia. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1449–1453. doi: 10.1073/pnas.80.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave T. G. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970 Mar;49(3):465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steender S., Zilversmit D. B. Arterial influx of esterified cholesterol from two plasma lipoprotein fractions and its hydrolysis in vivo in hypercholesterolemic rabbits. Atherosclerosis. 1981 Apr;39(1):97–109. doi: 10.1016/0021-9150(81)90092-7. [DOI] [PubMed] [Google Scholar]
- Stender S., Zilversmit D. B. Comparison of cholesteryl ester transfer from chylomicrons and other plasma lipoproteins to aortic intima media of cholesterol-fed rabbits. Arteriosclerosis. 1982 Nov-Dec;2(6):493–499. doi: 10.1161/01.atv.2.6.493. [DOI] [PubMed] [Google Scholar]
- Thompson K. H., Zilversmit D. B. Plasma very low density lipoprotein (VLDL) in cholesterol-fed rabbits: chylomicron remnants or liver lipoproteins? J Nutr. 1983 Oct;113(10):2002–2010. doi: 10.1093/jn/113.10.2002. [DOI] [PubMed] [Google Scholar]
- Weintraub M. S., Eisenberg S., Breslow J. L. Dietary fat clearance in normal subjects is regulated by genetic variation in apolipoprotein E. J Clin Invest. 1987 Dec;80(6):1571–1577. doi: 10.1172/JCI113243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub M. S., Eisenberg S., Breslow J. L. Different patterns of postprandial lipoprotein metabolism in normal, type IIa, type III, and type IV hyperlipoproteinemic individuals. Effects of treatment with cholestyramine and gemfibrozil. J Clin Invest. 1987 Apr;79(4):1110–1119. doi: 10.1172/JCI112926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub M. S., Rosen Y., Otto R., Eisenberg S., Breslow J. L. Physical exercise conditioning in the absence of weight loss reduces fasting and postprandial triglyceride-rich lipoprotein levels. Circulation. 1989 May;79(5):1007–1014. doi: 10.1161/01.cir.79.5.1007. [DOI] [PubMed] [Google Scholar]
- Weintraub M. S., Zechner R., Brown A., Eisenberg S., Breslow J. L. Dietary polyunsaturated fats of the W-6 and W-3 series reduce postprandial lipoprotein levels. Chronic and acute effects of fat saturation on postprandial lipoprotein metabolism. J Clin Invest. 1988 Dec;82(6):1884–1893. doi: 10.1172/JCI113806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub M., Ringel Y., Rassin T., Arad J., Bodner G., Liron M. Octogenarian subjects have low postprandial levels of chylomicron remnants: a possible explanation for protection against atherosclerosis. J Gerontol. 1992 Nov;47(6):B209–B213. doi: 10.1093/geronj/47.6.b209. [DOI] [PubMed] [Google Scholar]
- Wilson D. E., Chan I. F., Ball M. Plasma lipoprotein retinoids after vitamin A feeding in normal man: minimal appearance of retinyl esters among low-density lipoproteins. Metabolism. 1983 May;32(5):514–517. doi: 10.1016/0026-0495(83)90016-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. E., Chan I. F., Buchi K. N., Horton S. C. Postchallenge plasma lipoprotein retinoids: chylomicron remnants in endogenous hypertriglyceridemia. Metabolism. 1985 Jun;34(6):551–558. doi: 10.1016/0026-0495(85)90193-3. [DOI] [PubMed] [Google Scholar]
- Zilversmit D. B. Atherogenesis: a postprandial phenomenon. Circulation. 1979 Sep;60(3):473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]


