Abstract
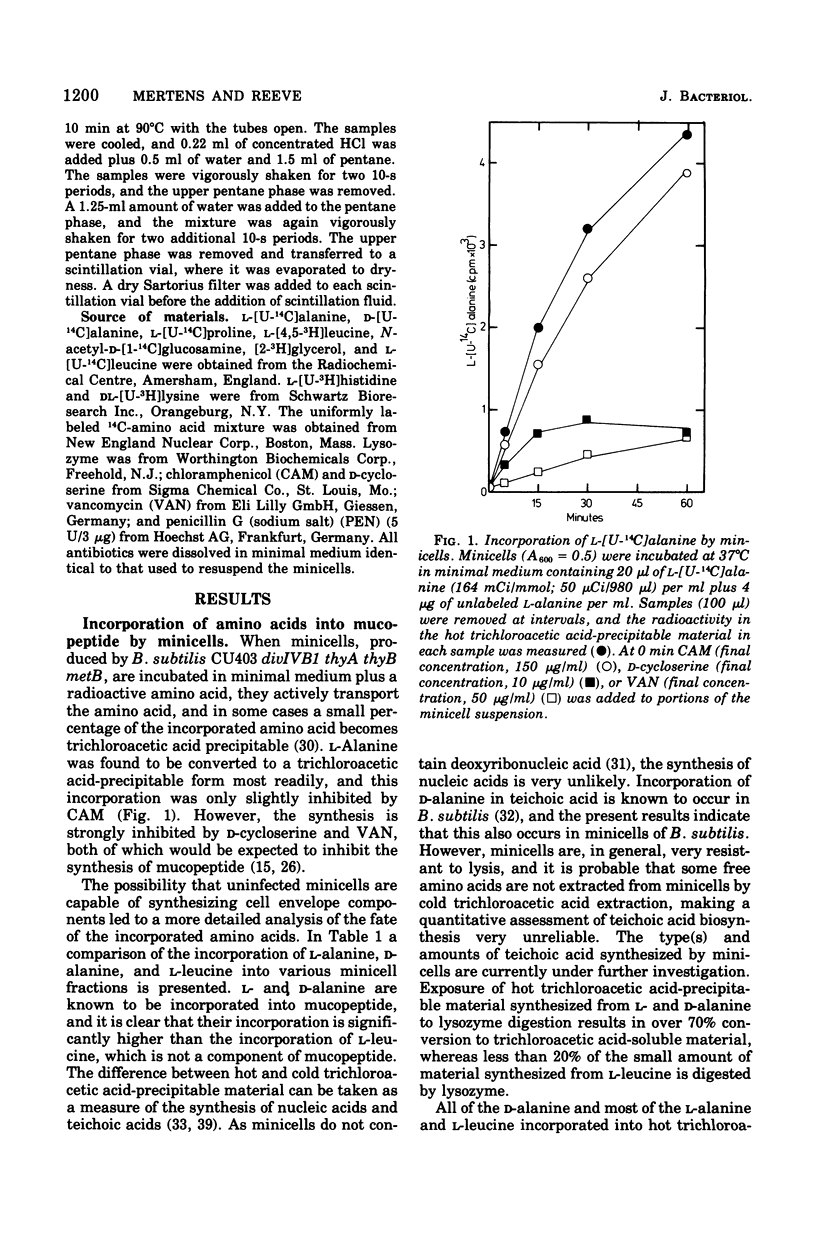
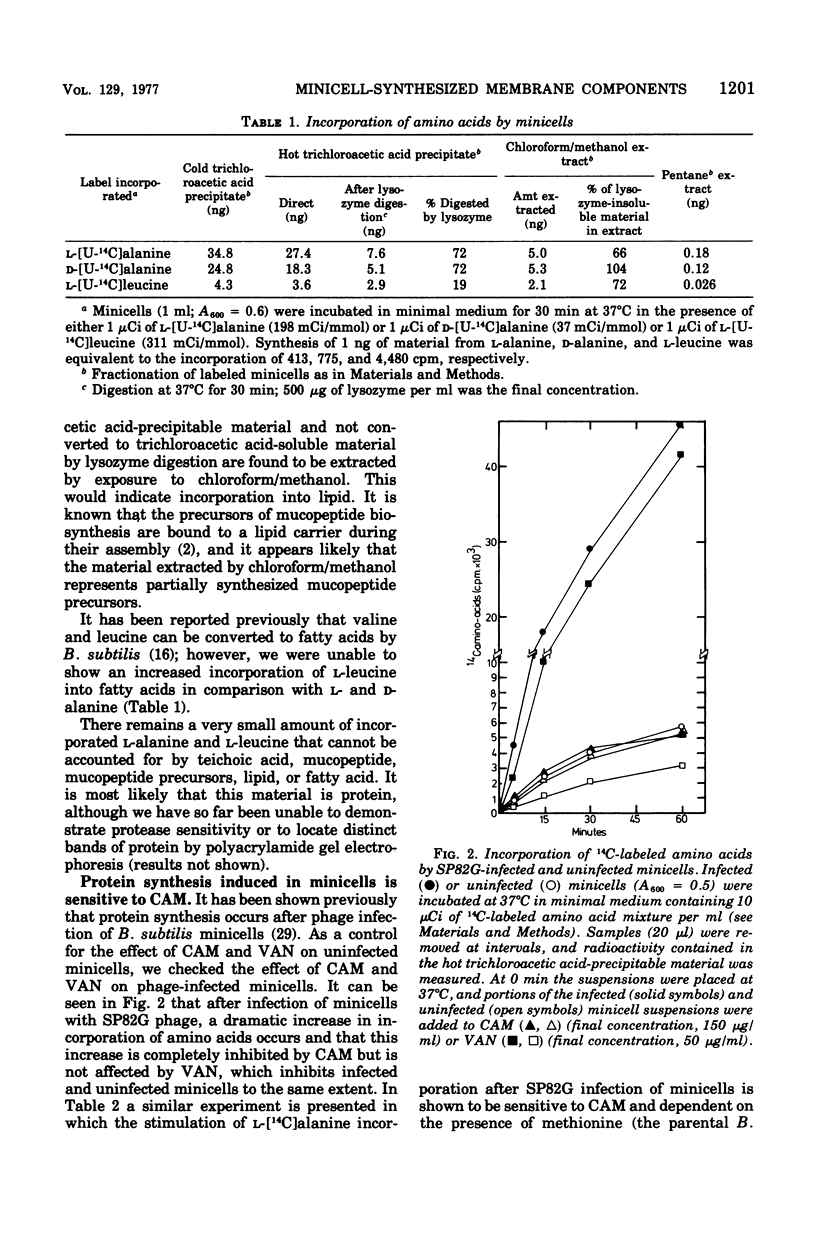
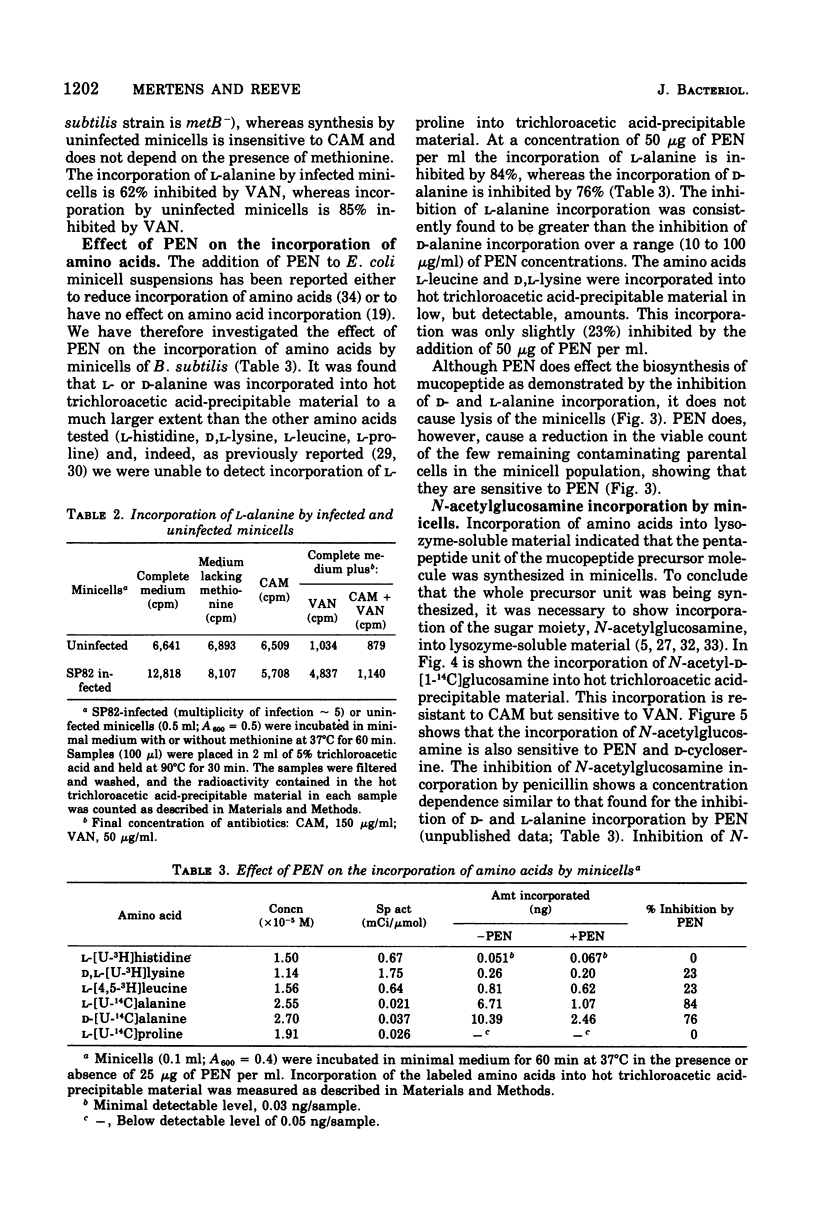
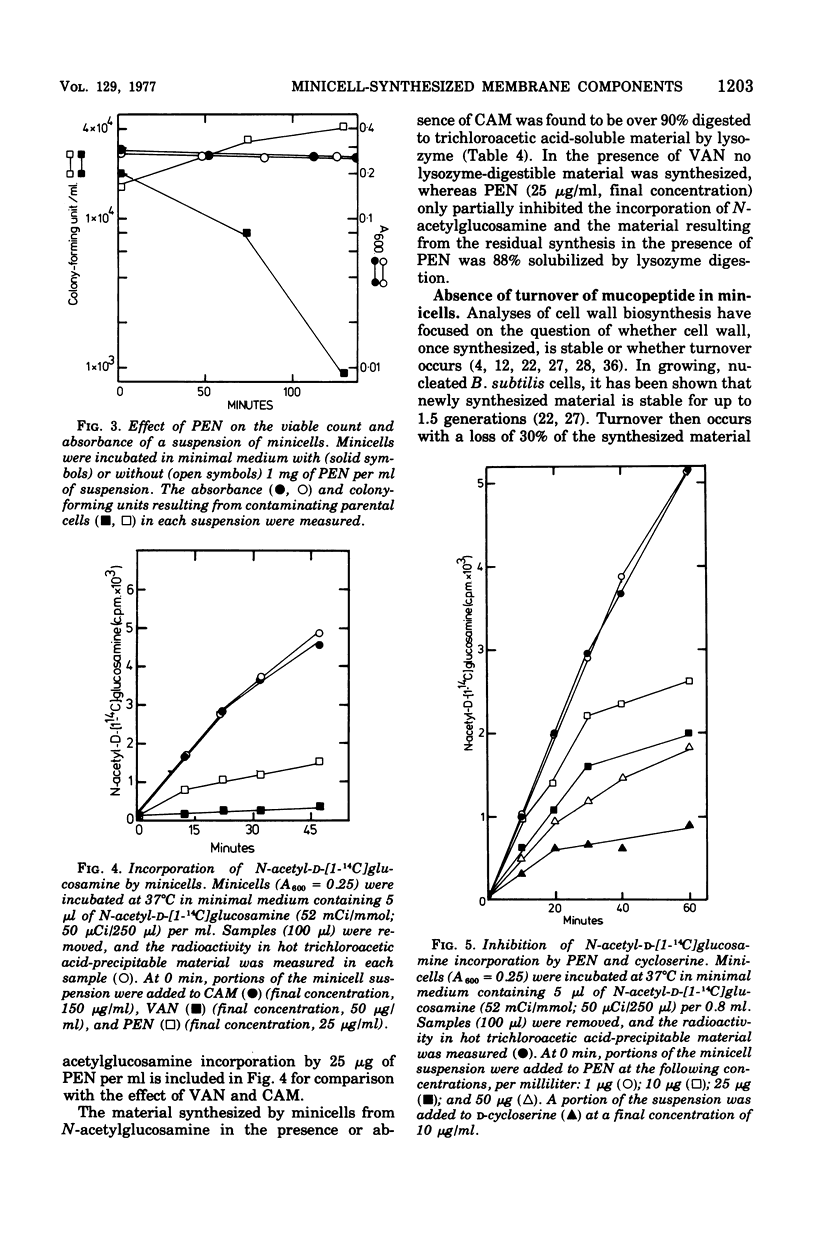
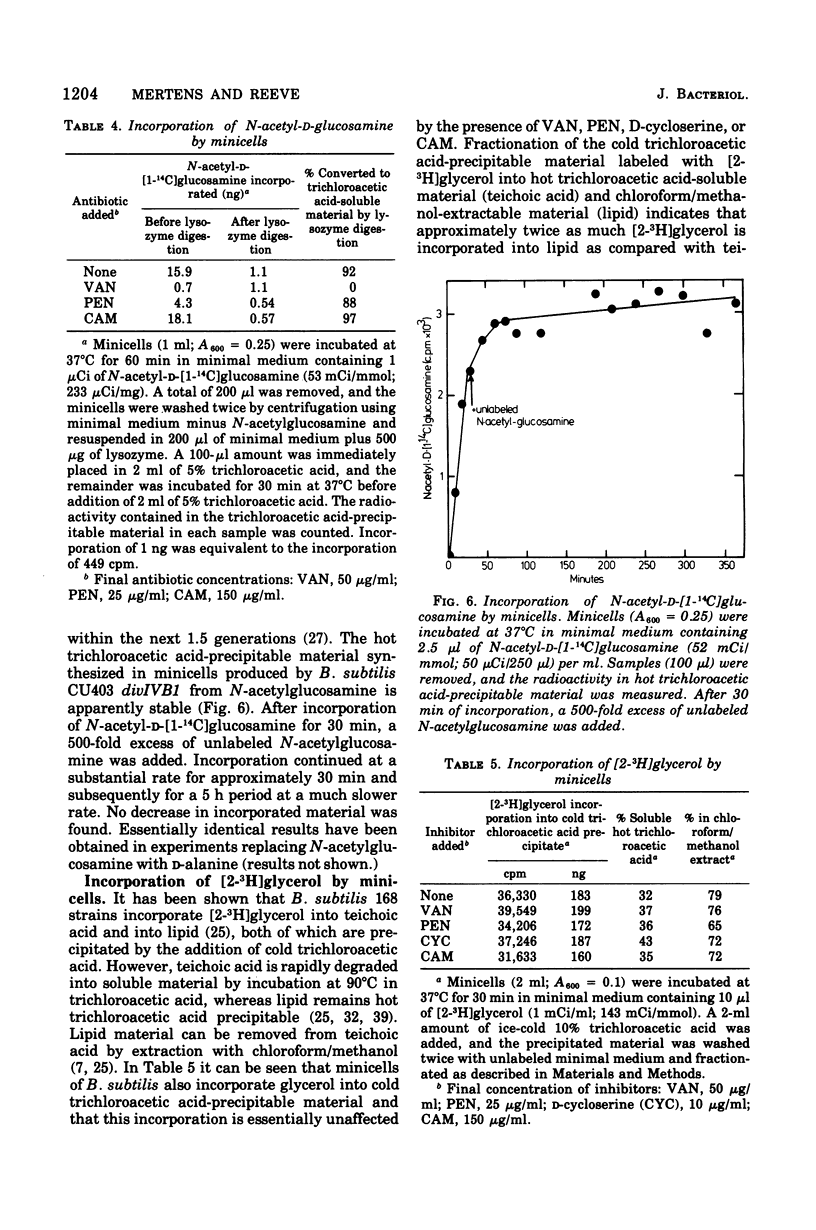
Minicells produced by Bacillus subtilis CU403 (divIVB1) are capable of mucopeptide biosynthesis as shown by the incorporation of L-alanine, D-alanine, and N-acetylglucosamine into trichloroacetic acid-precipitable material, which can be degraded to trichloroacetic acid-soluble material by lysozyme digestion. Incorporation of the precursors is sensitive to vancomycin and D-cycloserine and insensitive to chloramphenicol. Penicillin inhibits the incorporation of D- and L-alanine N-acetylglucosamine at concentrations in excess of 10 mug of penicillin per ml; however, minicells are insensitive to penicillin-induced lysis. The material synthesized in minicells from N-acetylglucosamine is not subject to turnover during a subsequent 6-h incubation period. [2-3H]glycerol is converted to a cold trichloroacetic acid-precipitable form by minicells. This synthesis is not inhibited by vancomycin, penicillin, D-cycloserine, or chloramphenicol. Fractionation of the material synthesized from glycerol into hot trichloroacetic acid-soluble material and chloroform/methanol-extractable material indicates that minicells convert glycerol into teichoic acid and lipid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. G., Rutberg L., Samuelsson B. The chemical composition of the cytoplasmic membrane of Bacillus subtilis. Eur J Biochem. 1967 Nov;2(4):448–453. doi: 10.1111/j.1432-1033.1967.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Duckworth M., Archibald A. R., Baddiley J. The location of N-acetylgalactosamine in the walls of Bacillus subtilis 168. Biochem J. 1972 Dec;130(3):691–696. doi: 10.1042/bj1300691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E. Structural difference between walls from hemispherical caps and partial septa of Bacillus subtilis. J Bacteriol. 1973 May;114(2):790–797. doi: 10.1128/jb.114.2.790-797.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Derepression of anthranilate synthase in purified minicells of Escherichia coli containing the Col-trp plasmid. J Bacteriol. 1973 Aug;115(2):615–622. doi: 10.1128/jb.115.2.615-622.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- HASH J. H., WISHNICK M., MILLER P. A. ON THE MODE OF ACTION OF THE TETRACYCLINE ANTIBIOTICS IN STAPHYLOCOCCUS AUREUS. J Biol Chem. 1964 Jun;239:2070–2078. [PubMed] [Google Scholar]
- Hash J. H., Davies M. C. Electron Microscopy of Staphylococcus aureus Treated with Tetracycline. Science. 1962 Nov 16;138(3542):828–829. doi: 10.1126/science.138.3542.828. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Early changes in the ultrastructure of Streptococcus faecalis after amino acid starvation. J Bacteriol. 1970 Jul;103(1):244–253. doi: 10.1128/jb.103.1.244-253.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Takata R., Muto A., Osawa S. Ribosomal RNA synthesis in the F'14 episome-containing minicells of Escherichia coli. Mol Gen Genet. 1974;128(4):341–347. doi: 10.1007/BF00268521. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Tanner P. J., Stokes E. Cell-wall thickening in Bacillus subtilis. Comparison of thickened and normal walls. Biochem J. 1970 Nov;120(1):159–170. doi: 10.1042/bj1200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEDA T. Valine as a source of the branched short chain precursor in the biosynthesis if iso-C14, iso-C15, iso-C16 and iso-C17 fatty acids by Bacillus subtitlis. Biochem Biophys Res Commun. 1963 Feb 6;10:283–287. doi: 10.1016/0006-291x(63)90431-5. [DOI] [PubMed] [Google Scholar]
- Kool A. J., van Zeben M. S., Nijkamp H. J. Identification of messenger ribonucleic acids and proteins synthesized by the bacteriocinogenic factor Clo DF13 in purified minicells of Escherichia coli. J Bacteriol. 1974 Apr;118(1):213–224. doi: 10.1128/jb.118.1.213-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., McMurry L., Palmer E. R factor proteins synthesized in Escherichia coli minicells: membrane-associated R factor proteins. J Bacteriol. 1974 Dec;120(3):1464–1471. doi: 10.1128/jb.120.3.1464-1471.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. Physical and functional characteristics of R-factor deoxyribonucleic acid segregated into Escherichia coli minicells. J Bacteriol. 1971 Oct;108(1):300–308. doi: 10.1128/jb.108.1.300-308.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. R factor proteins synthesized in Escherichia coli minicells: incorporation studies with different R factors and detection of deoxyribonucleic acid-binding proteins. J Bacteriol. 1974 Dec;120(3):1451–1463. doi: 10.1128/jb.120.3.1451-1463.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. Very stable prokaryotic messenger RNA in chromosomeless Escherichia coli minicells. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2900–2904. doi: 10.1073/pnas.72.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Mendelson N. H., Reeve J. N., Cole R. M. Physiological studies of Bacillus subtilis minicells. J Bacteriol. 1974 Mar;117(3):1312–1319. doi: 10.1128/jb.117.3.1312-1319.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. I. Isolation and properties of strains bearing mutations in glycerol metabolism. J Mol Biol. 1970 Apr 28;49(2):415–432. doi: 10.1016/0022-2836(70)90254-8. [DOI] [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Cornett J. B. Bacteriophage SPO1-induced macromolecular synthesis in minicells of Bacillus subtilis. J Virol. 1975 Jun;15(6):1308–1316. doi: 10.1128/jvi.15.6.1308-1316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H. Minicells of Bacillus subtilis. A unique system for transport studies. Biochim Biophys Acta. 1974 Jun 13;352(2):298–306. doi: 10.1016/0005-2736(74)90221-1. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F., Taylor C., Reeve J. N. Mucopeptide synthesis by rod mutants of Bacillus subtilis. J Gen Microbiol. 1974 Dec;85(2):335–349. doi: 10.1099/00221287-85-2-335. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick B., Setlow J. K., Boling M. E., Allison D. P. Minicell production and bacteriophage superinducibility of thymidine-requiring strains of Haemophilus influenzae. J Bacteriol. 1975 Sep;123(3):1208–1217. doi: 10.1128/jb.123.3.1208-1217.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Suginaka H., Blumberg P. M., Strominger J. L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5279–5288. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vambutas V. K., Salton M. R. Differential inhibitory effects of chloramphenicol on the synthesis of membrane ATPase and cytoplasmic enzymes of Micrococcus lysodeikticus. Biochim Biophys Acta. 1970 Mar 17;203(1):94–103. doi: 10.1016/0005-2736(70)90039-8. [DOI] [PubMed] [Google Scholar]
- Vörös J., Goodman R. N. Filamentous forms of Erwinia amylovora. Phytopathology. 1965 Aug;55(8):876–879. [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewicz H., Taylor K. Ribonucleic acid synthesis after adsorption of the bacteriophage lambda on Escherichia coli minicells. Acta Microbiol Pol A. 1975;7(1):21–24. [PubMed] [Google Scholar]
- van Embden J., Cohen S. N. Molecular and genetic studies of an R factor system consisting of independent transfer and drug resistance plasmids. J Bacteriol. 1973 Nov;116(2):699–709. doi: 10.1128/jb.116.2.699-709.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


