Abstract
Thyrotropin-releasing hormone (TRH) is a brain hypothalamic hormone that regulates thyrotropin (TSH) secretion from the anterior pituitary and is ubiquitously distributed throughout the brain and other tissues including pancreas. To facilitate studies into the role of endogenous TRH, we have used homologous recombination to generate mice that lack TRH. These TRH−/− mice are viable, fertile, and exhibit normal development. However, they showed obvious hypothyroidism with characteristic elevation of serum TSH level and diminished TSH biological activity. Their anterior pituitaries exhibited an apparent decrease in TSH immunopositive cells that was not due to hypothyroidism. Furthermore, this decrease could be reversed by TRH, but not thyroid hormone replacement, suggesting a direct involvement of TRH in the regulation of thyrotrophs. The TRH−/− mice also exhibited hyperglycemia, which was accompanied by impaired insulin secretion in response to glucose. These findings indicate that TRH−/− mice provide a model of exploiting tertiary hypothyroidism, and that TRH gene abnormalities cause disturbance of insulin secretion resulting in marked hyperglycemia.
Thyrotropin-releasing hormone (TRH) was originally isolated as the first hypothalamic hormone (1, 2) and has been shown to be present in many organs, including the central nervous system, reproductive system, and gastrointestinal tract (3, 4). Administration of exogenous TRH to animals and humans has been observed to cause a variety of endocrine and nonendocrine biological activities (3–5). The results of these studies have led to the suggestion that TRH may act as a neurotransmitter or neuromodulator in many organs.
Because of the ubiquitous distribution of TRH in many organs and the lack of a suitable method to produce selective changes in in vivo TRH level, it has been difficult to examine the mechanism by which TRH regulates different biological activities. For example, TRH is the major regulator of synthesis and secretion of thyrotropin (TSH) in the anterior pituitary and, thus, it plays a pivotal role in the regulation of the hypothalamic–pituitary–thyroid axis. However, the contribution of TRH in the functional maturation of pituitary thyrotrophs during development remains obscure. Second, although isolated deficiency of TRH has been suggested to be the cause of tertiary (hypothalamic) hypothyroidism, there is neither direct clinical evidence nor an animal model to support this hypothesis. In the pancreas, TRH exists in the islets of Langerhans and more specifically within the β cells producing insulin (6–9). However, due to technical difficulties associated with selective depletion of TRH in the β cells, it has been difficult to discern its role in insulin physiology.
In view of the aforementioned observations, it is clear that the availability of a TRH-deficient animal model will greatly enhance our ability to clarify the precise role of the peptide in many organ functions.
We recently cloned and characterized a mouse hypothalamic cDNA and genomic DNA encoding preproTRH (pTRH), and found that the mouse pTRH has a characteristic structure containing five repetitive copies of the TRH progenitor sequence (10, 11). These recent cloning studies have facilitated the use of a molecular genetic approach to determine the physiological functions of TRH. In this study, we have used gene targeting in mouse embryonic stem (ES) cells to generate mice deficient in TRH.
MATERIALS AND METHODS
Animals.
Procedures of animal care and use in this study were approved by the Review Committee on Animal Use at Gunma University (Maebashi, Japan). Animals were maintained on a 12-hr light/12-hr dark schedule (light on at 0600) and fed laboratory chow and tap water ad libitum. All experiments were done between 0900 and 1100. The mice with thyroxin (T4) replacement received 1.2 μg T4/100 g body weight subcutaneously for 10 days before the experiment. TRH replacement in TRH−/− mice was achieved by administrating 1.0 mg/kg per day of TRH through a continuous mini-osmotic pump over a period of 14 days. Experimental hypothyroid mice were generated by providing drinking water containing 0.02% 2-mercapto-1-methyl-imidazole (MMI) for 25 days.
Construction of the Targeting Vector.
The mouse pTRH gene was isolated from a TT2 genomic DNA library using a mouse pTRH cDNA as a probe (12). The 6.3-kb SalI–XbaI fragment of the pTRH gene, which included first and second exons and the 5.3-kb 5′-flanking sequence was inserted at the 5′ end of the cassette for the neomycin-resistance gene with no polyadenylation signal under the control of Pgk promoter (13). A short fragment (0.8 kb), containing the 3′ portion of the coding sequence and the 3′ untranslated region, was amplified by PCR. This fragment was then subcloned at the 3′ end of the above construct. A negative selection marker, diphtheria-toxin α subunit cassette, was also placed at the 3′ end of the vector.
Isolation of Targeted ES Cell Lines.
TT2 cells, derived from (C57BL/6 × CBA) F1 embryos, were transfected with a linearized targeting vector by electroporation (14). The medium was changed after 24 hr, and selection was applied 48 hr later by the addition of 200 μg/ml G418. On day 8, the PCR positive clones were expanded for isolation of genomic DNA for Southern blot analysis. The targeting frequency at the pTRH locus was 1/12.
Generation of Chimeric Mice.
Two independent ES cell clones that carried a targeted allele were injected into eight-cell ICR embryos and transferred into pseudopregnant ICR foster mothers as described (15). Chimeric males were then mated with ICR and C57BL/6 females. Mice bearing the targeted pTRH allele were interbred and F2 offspring were analyzed further by PCR and Southern blotting.
RNA Analysis.
Total RNA (20 μg) extracted from the hypothalamus was resolved through a 1.2% formaldehyde agarose gel and transferred onto a nylon membrane (16). The membrane was hybridized under high stringency conditions with a 265-bp pTRH cRNA probe, which encompassed the region between 307 and 572 bp from the translational start site.
Radioimmunoassay (RIA).
Hypothalamic TRH content was measured by a specific RIA as described (17). Serum prolactin (PRL), growth hormone (GH), T4, and triiodothyronine (T3) were determined by double-antibody RIAs with reagents provided by A. F. Parlow (Harbor–University of California at Los Angeles Medical Center, Torrance, CA) (mouse PRL); the National Pituitary Agency, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (rat GH); and Dainabot (Tokyo) (T4 and T3). TSH was measured by a specific mouse TSH RIA with mouse TSH/LH reference (AFP51718MP), mouse TSH antiserum (AFP98991), and rat TSH antigen (NIDDK-rTSH-I-9), all of which were obtained from A. F. Parlow. The detection limit of the assay was 15 ng TSH/ml. The intra-assay variation was less than 6%, and all samples were measured in one series to prevent inter-assay variation. The TSH RIA used here was observed to detect the authentic TSH peak (Mr = ≈28 kDa), but not a TSHα subunit peak (Mr = ≈12 kDa), of the pituitary extracts on an HPLC gel filtration chromatography Zorbax GF-250 column (9.4 × 250 mm, DuPont).
TRH and TSH Stimulation Tests.
Serum TSH (30 min after injection) and T3 (120 min after injection) levels were determined before and after TRH injection (5.0 μg/kg, i.p.) in the mice. T3 levels were determined 120 min after bovine TSH (Sigma) administration (250 milliunits/100 g, i.p.).
Glucose Tolerance Test.
The mice were fasted for 16 hr before the study and were then loaded with 2.0 g/kg glucose i.p. Blood glucose and plasma insulin levels were successively measured. Immunoreactive insulin concentrations in plasma were measured by RIA (Pharmacia) with rat insulin as a standard.
Insulin Tolerance Test.
The mice were fasted during the study. Human insulin (Eli Lilly, 0.75 unit/kg, i.p.) was injected and blood glucose levels were measured.
Batch Incubation Study.
Batches of five islets were preincubated for 60 min at 37°C in 1 ml of Hanks’ buffered saline containing 10 mM Hepes (pH 7.5) and 0.1% BSA plus 0.1 mM glucose. The medium was then replaced with 1 ml of buffer supplemented with secretagogues. After 60 min incubation at 37°C, the medium was removed for insulin RIA.
Insulin Content Assay.
Five isolated islets were suspended and homogenized in 500 μl of 0.1 M HCl, and cellular insulin was then extracted, diluted (1:100), and assayed by RIA.
Immunohistochemistry.
Pituitary and pancreatic sections were fixed in 10% formalin and embedded in paraffin wax. Rabbit anti-rat TSH β-IC-1 (AFP1274789, NIDDK), anti-human insulin, and anti-human glucagon antibodies (MILAB, Malmoe, Sweden) were applied to the sections, and their staining was visualized by a biotin–streptavidin method.
Ultrastructural Study.
Pancreatic tissues were fixed in 1% glutaraldehyde and 4% formaldehyde, postfixed in 2% osmic acid, and embedded in Quetol 812 (Nisshin EM, Tokyo). Ultrathin sections were stained with acetate.
Statistical Analysis.
All data were analyzed by variant analysis and Duncan’s multiple test.
RESULTS
Targeted Disruption of the pTRH Gene.
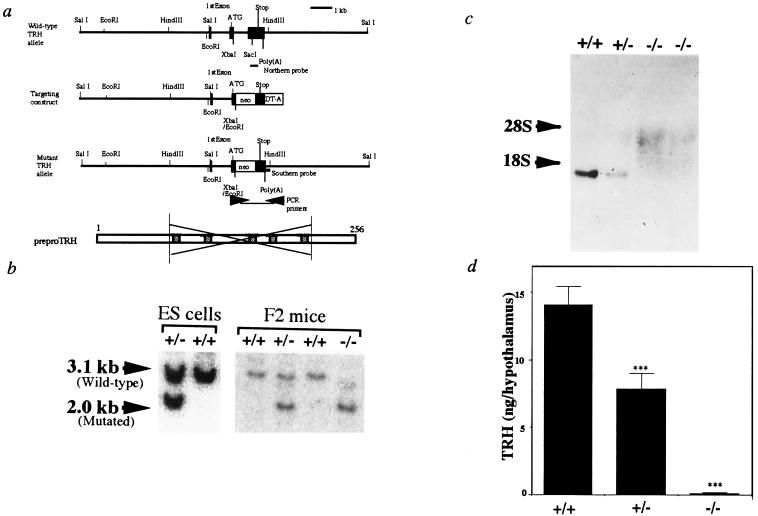
The region deleted by homologous recombination contained codons 70–211 including all 5 copies of the TRH progenitor sequence (Fig. 1a). Male chimeras transmitted the mutant pTRH allele to their offspring. The heterozygotes (TRH+/−) were apparently normal, and subsequently gave rise to mice homozygous (TRH−/−) for the mutant pTRH locus (Fig. 1b). Northern blot analysis showed no hybridizing transcript in the TRH−/− mice (Fig. 1c), confirming disruption of the pTRH gene. No TRH immunoreactivity was found in the TRH−/− mice hypothalami (Fig. 1d).
Figure 1.
Targeted disruption of the pTRH gene. (a) Schematic representation of the mouse pTRH gene (Upper), targeting vector, expected targeted allele, and pTRH (Lower). Solid boxes represent exons. Allele-specific PCR primers for selection of homologous recombinants are indicated by arrows, and probes for Southern and Northern blot analyses are also indicated. Hatched bars represent TRH progenitor sequences in pTRH. (b) Southern blot analysis of DNA from ES cell clones and siblings generated by crossing heterozygous mice (F2 mice). The wild-type (3.1 kb) and targeted (2.0 kb) HindIII and EcoRI fragments were detected by the 3′ external probe indicated in a. (c) Analysis of pTRH mRNA expression by Northern blot analysis of F2 mice. Total RNAs (20 μg) from the hypothalamus of wild-type (+/+), heterozygous (+/−), and homozygous mice (−/−) were hybridized with pTRH cRNA. (d) Hypothalamic TRH contents of the wild-type (+/+, n = 9), heterozygous (+/−, n = 5), and homozygous (−/−, n = 10) mice. ∗∗∗, P < 0.001 vs. wild type.
General Appearance, Fertility, Body Weight, and Histology.
Mutant mice were born at an approximately expected Mendelian frequency and were viable through adulthood. Initial inspection of the TRH−/− mice revealed no gross anatomical or functional abnormalities. No abnormalities were found on histological examination of the brain, retina, thyroid, heart, liver, spleen, kidney, gastrointestinal tract, or reproductive systems. Both TRH−/− females and males were fertile. One of the phenotypic characteristics observed in TRH−/− mice was that their growth was slightly retarded at the age of 4 weeks, corresponding to 1 week after weaning (Table 1). However, by 8 weeks of age there was no significant difference in body weight between the TRH+/+ and TRH−/− mice (Table 1). This transient growth retardation was restored by T4 replacement.
Table 1.
Body weights (g) and serum pituitary hormone levels
| Wild type | Heterozygous | Homozygous | |
|---|---|---|---|
| Body weight (4 weeks, male) | 20.8 ± 0.75 (7) | 20.2 ± 0.48 (8) | 14.5 ± 0.47 (10)*** |
| Body weight (8 weeks, male) | 29.0 ± 0.4 (10) | 29.4 ± 0.5 (19) | 28.1 ± 0.21 (12) NS |
| TSH (ng/ml) | 76.0 ± 3.5 (13) | 83.7 ± 4.4 (10) | 139.1 ± 7.3 (13)** |
| PRL (ng/ml) | 12.7 ± 1.8 (7) | 13.5 ± 3.2 (8) | 10.7 ± 2.2 (10) NS |
| GH (ng/ml) | 41.3 ± 3.2 (9) | 47.4 ± 4.5 (7) | 45.2 ± 9.7 (11) NS |
| T4 (μg/dl) | 5.94 ± 0.28 (13) | 5.54 ± 0.35 (8) | 3.30 ± 0.27 (13)*** |
Serum TSH, PRL, GH, and T4 levels were determined in 8-week-old male mice. Data represent means ± SEM (numbers of mice in parentheses). Significance of differences between wild-type and mutant animals is indicated: ∗∗∗, P < 0.001; ∗∗, P < 0.01; NS, not significant.
Analysis of the Pituitary Functions.
In the TRH−/− mice, serum GH and PRL levels were normal, whereas T4 levels were decreased to approximately 60% of those in the TRH+/+ and TRH+/− mice (Table 1). TRH replacement increased T4 levels to the normal (5.9 ± 0.5 μg/dl, n = 7). In both the wild-type and mutant mice, MMI treatment induced decreases in T4 levels (TRH+/+, 2.48 ± 0.08, n = 5; TRH−/−, 1.57 ± 0.09 μg/dl, n = 5) and increases in TSH levels (157.6 ± 8.2 and 194.0 ± 18.5 ng/ml), indicating that the feedback regulation of TSH secretion by thyroid hormone worked at least in part in the TRH−/− mice.
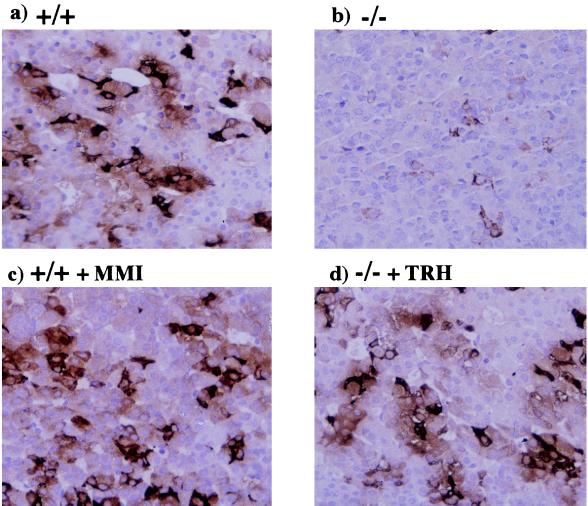
Immunohistochemical analysis of the pituitary in TRH−/− mice showed that the numbers of TSH positive cells were significantly decreased and could be restored by TRH, but not by thyroid hormone replacement (Fig. 2 a, b, and d). Moreover, hypothyroidism induced by MMI treatment did not cause apparent morphological changes in pituitaries of the wild-type (Fig. 2c) or TRH−/− mice (data not shown).
Figure 2.
Immunohistochemical analysis of the pituitary. When compared with those of the wild type (a), numbers of TSH positive cells in the TRH−/− pituitary (b) were decreased. This decrease was restored by TRH replacement (d). No significant change was found in the pituitary of the hypothyroid mice induced by MMI treatment (c).
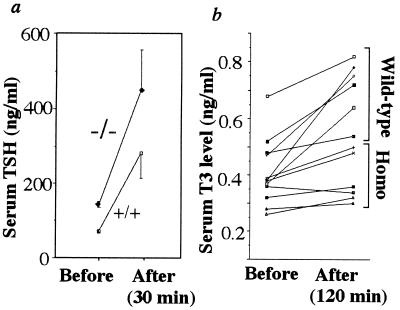
Although a decrease in serum TSH levels was expected in the TRH−/− mice, their levels of serum TSH were approximately 2-fold higher than those in the TRH+/+ and TRH+/− littermates (Table 1). This elevated TSH was further stimulated by TRH injection (Fig. 3a). The successive response of T3 was significantly impaired in the mutant mice (TRH+/+, 51.6 ± 14.2; TRH−/−, 16.1 ± 4.6% increment over the basal, P < 0.05, Fig. 3b). In contrast, stimulation of T3 secretion after exogenous TSH administration was similar in the TRH+/+ and TRH−/− mice (39.5 ± 4.5, n = 6 vs. 45.2 ± 6.6% increment over the basal, n = 5).
Figure 3.
TRH stimulation test. After TRH administration, blood TSH (a) and T3 levels (b) were determined in the wild-type (+/+, n = 6) and homozygous (−/−, n = 6) mice. The increment in T3 over the basal level was reduced in the homozygotes.
Blood Glucose Levels and Insulin Secretion.
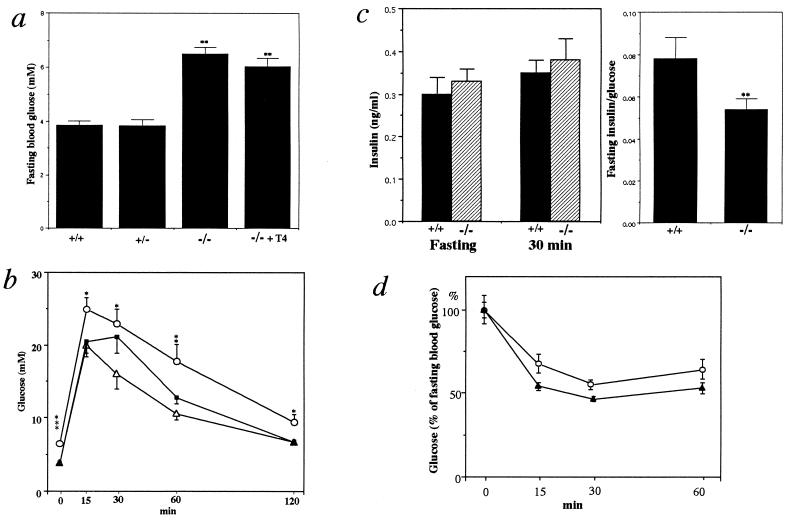
To our surprise, despite the lack of any apparent anatomical and physiological differences, fasting blood glucose levels of the TRH−/− mice were markedly higher than those of the TRH+/+ and TRH+/− mice (TRH+/+, 3.85 ± 0.17, n = 11; TRH−/−, 6.49 ± 0.24 mM, n = 10; Fig. 4a). T4 replacement to the TRH−/− mice did not correct this hyperglycemia, excluding the possibility that hyperglycemia was due to the concurrent hypothyroidism (Fig. 4a). Unfortunately, we could not assess blood glucose levels after TRH administration because of body weight loss due to implantation of the osmotic pump. Even after glucose challenge, the TRH−/− mice showed significantly elevated blood glucose levels (Fig. 4b). Consequently, the plasma insulin levels in these mice were low relative to the elevated blood glucose concentrations (Fig. 4c). The TRH−/− mice were as sensitive to insulin administration as the wild type (Fig. 4d). These results suggest that hyperglycemia in the TRH−/− mice mainly resulted from impaired insulin secretion in response to glucose.
Figure 4.
Blood glucose and plasma insulin levels in TRH−/− mice. (a) Fasting blood glucose levels in the wild-type (+/+, n = 11), heterozygous (+/−, n = 10), homozygous (−/−, n = 10) mice, and homozygous mice with T4 replacement (−/− + T4, n = 7). ∗∗, P < 0.01 vs. the wild-type and heterozygous mice. (b) Glucose tolerance tests. Blood glucose levels were determined after glucose challenge. Data indicate means ± SEM (n = 10–15). (▵), wild type; (▪), heterozygotes; (○), homozygotes. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. wild type. (c) Plasma insulin levels before and 30 min after glucose challenge, and the ratio of fasting plasma insulin to glucose. +/+, wild type, n = 7; −/−, homozygotes, n = 7. ∗∗, P< 0.01 vs. wild type. (d) Insulin tolerance tests. Results are presented as the percentage of the fasting blood glucose and are means ± SEM. (○), wild type, n = 5; (▴), homozygotes, n = 5.
Morphological and in Vitro Study of the Pancreatic Islets.
Immunohistochemical and electron microscopic analyses showed that islets from the TRH−/− mice contained normal α, β, and δ cells with numerous secretory granules (data not shown). In addition, the islet insulin contents were similar in the TRH+/+ and TRH−/− mice (44.9 ± 6.5, n = 15 vs. 47.2 ± 7.6 ng per 5 islets, n = 15).
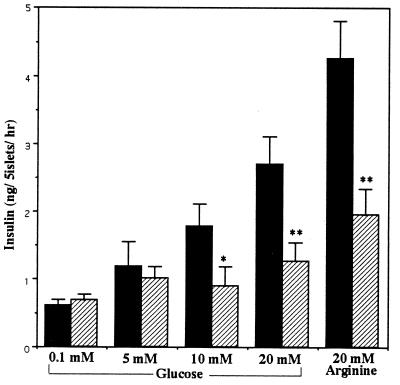
Whereas islet insulin secretion in vitro in response to 5 mM or less glucose did not differ between the TRH+/+ and TRH−/− mice, there was a significant decrease in insulin secretion in the mutant mice in response to 10 and 20 mM glucose (TRH+/+, 4.26 ± 0.55, n = 11; TRH−/−, 1.96 ± 0.38 ng per 5 islets/hr at 20 mM, n = 13, Fig. 5). Glucose (20 mM) is comparable to maximum blood glucose levels of animals after glucose challenge in vivo. The insulin secretory impairment was also observed in response to 20 mM arginine (Fig. 5). These results imply that the TRH−/− mice had marked dysfunction of insulin secretory response to increasing glucose concentrations.
Figure 5.
Characterization of TRH−/− islets. Insulin secretion in response to glucose and arginine was determined in vitro. Values are expressed as means ± SEM (n = 10–15). Solid bars, wild type; hatched bars, homozygotes. ∗, P < 0.05; ∗∗, P < 0.01 vs. wild type.
DISCUSSION
We have inactivated the pTRH gene by homologous recombination in ES cells; the resulting homozygous mutants completely lack TRH. Although TRH is known to be expressed during the early embryonic stages (18), the present studies show that its presence is not critical for embryonic survival or prenatal development. In addition, whereas TRH has been shown to exist in many organ systems (e.g., neuroendocrine, reproductive, gastrointestinal, as well as visual), its absence did not cause any apparent change in these systems.
Whereas the TRH−/− mice showed normal serum PRL and GH levels, thyroid hormone levels were significantly decreased compared with the wild-type and heterozygous mice. In the mutants, the number of pituitary TSH positive cells was decreased. This decrease, however, is not related to hypothyroidism in the TRH−/− mice, because MMI-induced hypothyroidism in the wild-type mice did not alter the number of TSH positive cells. Furthermore, the observation that this decrease could be reversed by TRH but not by thyroid hormone replacement indicates that the phenotypic change observed in the mutant pituitary was due to the direct effect of TRH deficiency rather than hypothyroidism. In addition, our data show that the presence of TRH is not required for normal development of thyrotrophs. In contrast, in previous animal experiments, induction of TRH deficiency by hypothalamic destruction with electrolytic lesion or knife-cut deafferentation has been shown to reduce blood TSH levels (19, 20). Although unexpected, blood TSH levels were elevated in the TRH−/− mice. There have been no patients with isolated TRH deficiency defined, but similar elevation has also been reported in some patients with central hypothyroidism (21). These patient’s TSH activities were observed to be significantly reduced (22, 23). Because the TRH−/− mice showed the diminished thyroid hormone response to endogenous TSH after TRH stimulation, but normal response to exogenous TSH, it is concluded that the biological activity of circulating TSH in the TRH−/− mice was attenuated. Therefore, the TRH−/− mice serve as the first animal model of tertiary hypothyroidism due to isolated TRH deficiency and establish that the novel entity of tertiary hypothyroidism involves increased rather than decreased blood TSH level. In light of this discussion, it is interesting to speculate that among patients diagnosed with primary hypothyroidism there may be a subset of patients with tertiary hypothyroidism, since both primary and tertiary hypothyroid conditions exhibit the identical finding of elevated blood TSH level with decreased thyroid hormone level.
Recently, a hypothyroid patient with compound heterozygous mutations of the gene encoding TRH receptor was reported (24). This patient showed a normal level of basal TSH and no response of TSH to TRH injection, an observation different from that in the TRH−/− mice. No mention was made of studies on glucose metabolism in this patient. This suggests that the hormonal status of TRH deficiency is distinguishable from that of TRH receptor dysfunction.
The TRH−/− mice showed marked hyperglycemia accompanied by impaired insulin secretion by islets after glucose challenge. TRH content in the pancreas has been reported to drastically change during the prenatal and postnatal periods (8, 9). This suggests that TRH deficiency may affect fetal development of the pancreas, but our observations did not support this hypothesis.
Secretory granules of pancreatic β cells have been shown to contain several biologically active peptides including TRH, amylin, and chromogranines (25–27). These peptides are colocalized with insulin within the same secretory granules and have been suggested to possess modulatory effects on insulin secretion, but there is as yet no direct evidence to support this concept. Several studies have attempted to clarify roles of TRH in the pancreas, but no universal consensus has yet been reached (28–31). Taking advantage of the TRH−/− mice, we showed that TRH plays a key role in insulin secretion and demonstrated that knockout mice are good animal models in which to examine physiological functions of hormones/peptides colocalized with insulin in the pancreatic β cells.
The mechanisms by which lack of TRH affects insulin secretion remain to be elucidated. Several animal models for diabetes mellitus have recently been reported using the isolated gene targeting method, including glucokinase-deficient and insulin-receptor-substrate-1-deficient mice (32–35). As disruption of these molecules is apparently different from the condition in this study (32–35), the impairment of insulin secretion found in the TRH−/− mice is likely to be involved in diverse mechanisms. In the TRH−/− mice, five copies of the TRH progenitor sequence and its intervening peptides were simultaneously disrupted. Although several studies have shown biological activities of the intervening peptides on pituitary hormones, the results are still contradictory (36–40), and pathological roles of the intervening peptides in development of hyperglycemia of the TRH−/− mice remain to be determined.
In conclusion, targeted disruption of the pTRH gene caused characteristic tertiary hypothyroidism and a marked decrease in insulin secretion resulting in profound hyperglycemia. These findings imply that TRH gene abnormalities are not only a cause of tertiary hypothyroidism but also a possible candidate for one of multiple factors involved in the pathogenesis of diabetes mellitus. Furthermore, the TRH-deficient mice can also be used to examine roles of central TRH in varying neurobehavioral functions, including sleep, anxiety, depression, learning, and memory.
Acknowledgments
We thank anonymous reviewers for critical comments on the manuscript, C. Prasad (Louisiana State University Medical Center, New Orleans) for excellent advise, H. Kobayashi for assistance with RIA (Gunma University), and M. Yokota (Gunma University) for assistance with the immunohistochemistry. This work was supported by Clinical Research Special Fund-1996 from Gunma University Hospital (Maebashi, Japan) (to M.M.) and a grant-in-aid from the Ministry of Education, Science, Sports and Culture of Japan (to M.Y.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: TRH, thyrotropin-releasing hormone; TSH, thyrotropin; pTRH, preproTRH; ES, embryonic stem; MMI, 2-mercapto-1-methyl-imidazole; RIA, radioimmunoassay; PRL, prolactin; GH, growth hormone.
References
- 1.Boler J, Enzmann F, Folkers K, Bowers C Y, Schally A V. Biochem Biophys Res Commun. 1969;37:705–710. doi: 10.1016/0006-291x(69)90868-7. [DOI] [PubMed] [Google Scholar]
- 2.Burgus R, Dunn T F, Desiderio D, Guillemin R. C R Hebd Acad Sci Seances D. 1969;269:1870–1873. [PubMed] [Google Scholar]
- 3.Jackson I M. N Engl J Med. 1982;306:145–155. doi: 10.1056/NEJM198201213060305. [DOI] [PubMed] [Google Scholar]
- 4.Morley J E. Life Sci. 1979;25:1539–1550. doi: 10.1016/0024-3205(79)90435-1. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf G. Ann NY Acad Sci. 1989;553:311–313. [Google Scholar]
- 6.Kawano H, Daikoku S, Saito S. Endocrinology. 1983;112:951–955. doi: 10.1210/endo-112-3-951. [DOI] [PubMed] [Google Scholar]
- 7.Leduque P, Wolf B, Aratan-Spire S, Dubois P M, Czernichow P. Regul Pept. 1985;10:281–292. doi: 10.1016/0167-0115(85)90040-0. [DOI] [PubMed] [Google Scholar]
- 8.Leduque P, Aratan-Spire S, Czernichow P, Dubois P M. J Clin Invest. 1986;78:1028–1034. doi: 10.1172/JCI112657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martino E, Lernmark A, Seo H, Steiner D F, Refetoff S. Proc Natl Acad Sci USA. 1978;75:4265–4267. doi: 10.1073/pnas.75.9.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh T, Yamada M, Monden T, Iizuka M, Mori M. Mol Brain Res. 1992;14:131–135. doi: 10.1016/0169-328x(92)90020-c. [DOI] [PubMed] [Google Scholar]
- 11.Satoh T, Yamada M, Iwasaki T, Mori M. J Biol Chem. 1996;271:27919–27926. doi: 10.1074/jbc.271.44.27919. [DOI] [PubMed] [Google Scholar]
- 12.Yagi T, Nada S, Watanabe N, Tamemoto H, Kohmura N, Ikawa Y, Aizawa S. Anal Biochem. 1993;214:77–86. doi: 10.1006/abio.1993.1459. [DOI] [PubMed] [Google Scholar]
- 13.Boer P H, Potten H, Adra C N, Jardine K, Mullhofer G, McBurney M W. Biochem Genet. 1990;28:299–308. doi: 10.1007/BF02401419. [DOI] [PubMed] [Google Scholar]
- 14.Yagi T, Tokunaga T, Furuta Y, Nada S, Yoshida M, Tsukada T, Saga Y, Takeda N, Ikawa Y, Aizawa S. Anal Biochem. 1993;214:70–76. doi: 10.1006/abio.1993.1458. [DOI] [PubMed] [Google Scholar]
- 15.Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- 16.Yamada M, Monden T, Satoh T, Iizuka M, Murakami M, Iriuchijima T, Mori M. Biochem Biophys Res Commun. 1992;184:367–372. doi: 10.1016/0006-291x(92)91202-2. [DOI] [PubMed] [Google Scholar]
- 17.Mori M, Kobayashi I, Wakabayashi K. Metabolism. 1978;27:1485–1490. doi: 10.1016/s0026-0495(78)80020-1. [DOI] [PubMed] [Google Scholar]
- 18.Winters A J, Eskay R L, Porter J C. J Clin Endocrinol Metab. 1974;39:960–963. doi: 10.1210/jcem-39-5-960. [DOI] [PubMed] [Google Scholar]
- 19.Aizawa T, Kobayashi M, Komiya I, Hiramatsu K, Sato A, Yamada T. Endocrinology. 1984;114:8–13. doi: 10.1210/endo-114-1-8. [DOI] [PubMed] [Google Scholar]
- 20.Mori M, Yamada M, Kobayashi S. Neuroendocrinology. 1988;48:153–159. doi: 10.1159/000125003. [DOI] [PubMed] [Google Scholar]
- 21.Illig R, Krawczynska H, Torresani T, Prader A. J Clin Endocrinol Metab. 1975;41:722–728. doi: 10.1210/jcem-41-4-722. [DOI] [PubMed] [Google Scholar]
- 22.Beck-Peccoz P, Amr S, Menezes-Ferreira M M, Faglia G, Weintraub B D. N Engl J Med. 1985;312:1085–1090. doi: 10.1056/NEJM198504253121703. [DOI] [PubMed] [Google Scholar]
- 23.Faglia G, Bitensky L, Pinchera A, Ferrari C, Paracchi A, Beck-Peccoz P, Ambrosi B, Spada A. J Clin Endocrinol Metab. 1979;48:989–998. doi: 10.1210/jcem-48-6-989. [DOI] [PubMed] [Google Scholar]
- 24.Collu R, Tang J, Castagne J, Lagace G, Masson N, Huot C, Deal C, Delvin E, Faccenda E, Eidne K A, Vliet G V. J Clin Endocrinol Metab. 1997;82:1361–1365. doi: 10.1210/jcem.82.5.3918. [DOI] [PubMed] [Google Scholar]
- 25.Hutton J C, Penn E J, Peshavaria M. Diabetologia. 1982;23:365–373. doi: 10.1007/BF00253746. [DOI] [PubMed] [Google Scholar]
- 26.Madsen O D, Nielsen J H, Michelsen B, Westermark P, Betsholtz C, Nishi M, Steiner D F. Mol Endocrinol. 1991;5:143–148. doi: 10.1210/mend-5-1-143. [DOI] [PubMed] [Google Scholar]
- 27.Cetin Y, Aunis D, Bader M F, Galindo E, Jorns A, Bargsten G, Grube D. Proc Natl Acad Sci USA. 1993;90:2360–2364. doi: 10.1073/pnas.90.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awouters P, Meissner H P, Henquin J C. Diabetes Res. 1985;2:105–110. [PubMed] [Google Scholar]
- 29.Dolva L O, Hanssen K F, Flaten O, Hanssen L E, von Schenck H. Acta Endocrinol (Copenhagen) 1983;102:224–230. doi: 10.1530/acta.0.1020224. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni R N, Wang Z L, Akinsanya K O, Bennet W M, Wang R M, Smith D M, Ghatei M A, Byfield P G, Bloom S R. Endocrinology. 1995;136:5155–5164. doi: 10.1210/endo.136.11.7588254. [DOI] [PubMed] [Google Scholar]
- 31.Vara E, Tamarit-Rodriguez J. Acta Endocrinol (Copenhagen) 1988;118:429–436. doi: 10.1530/acta.0.1180429. [DOI] [PubMed] [Google Scholar]
- 32.Grupe A, Hultgren B, Ryan A, Ma Y H, Bauer M, Stewart T A. Cell. 1995;83:69–78. doi: 10.1016/0092-8674(95)90235-x. [DOI] [PubMed] [Google Scholar]
- 33.Terauchi Y, Sakura H, Yasuda K, Iwamoto K, Takahashi N, Ito K, Kasai H, Suzuki H, Ueda O, Kamada N, Jishage K, Komeda K, Noda M, Kanazawa Y, Taniguchi S, Miwa I, Akanuma Y, Kodama T, Yazaki Y, Kadowaki T. J Biol Chem. 1995;270:30253–30256. doi: 10.1074/jbc.270.51.30253. [DOI] [PubMed] [Google Scholar]
- 34.Araki E, Lipes M A, Patti M E, Bruning J C, Haag B, III, Johnson R S, Kahn C R. Nature (London) 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 35.Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, Sekihara H, Yoshioka S, Horikoshi H, Furuta Y, Ikawa Y, Kasuga M, Yazaki Y, Aizawa S. Nature (London) 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 36.Bulant M, Roussel J P, Astier H, Nicolas P, Vaudry H. Proc Natl Acad Sci USA. 1990;87:4439–4443. doi: 10.1073/pnas.87.12.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carr F E, Fein H G, Fisher C U, Wessendorf M W, Smallridge R C. Endocrinology. 1992;131:2653–2658. doi: 10.1210/endo.131.6.1446607. [DOI] [PubMed] [Google Scholar]
- 38.Redei E, Hilderbrand H, Aird F. Endocrinology. 1995;136:3557–3563. doi: 10.1210/endo.136.8.7628393. [DOI] [PubMed] [Google Scholar]
- 39.Mitsuma T, Hirooka Y, Kimura M, Nogimori T. Endocrinol Exp. 1990;24:333–339. [PubMed] [Google Scholar]
- 40.Nicholson W E, Orth D N. Endocrinology. 1996;137:2171–2174. doi: 10.1210/endo.137.5.8612564. [DOI] [PubMed] [Google Scholar]