Abstract
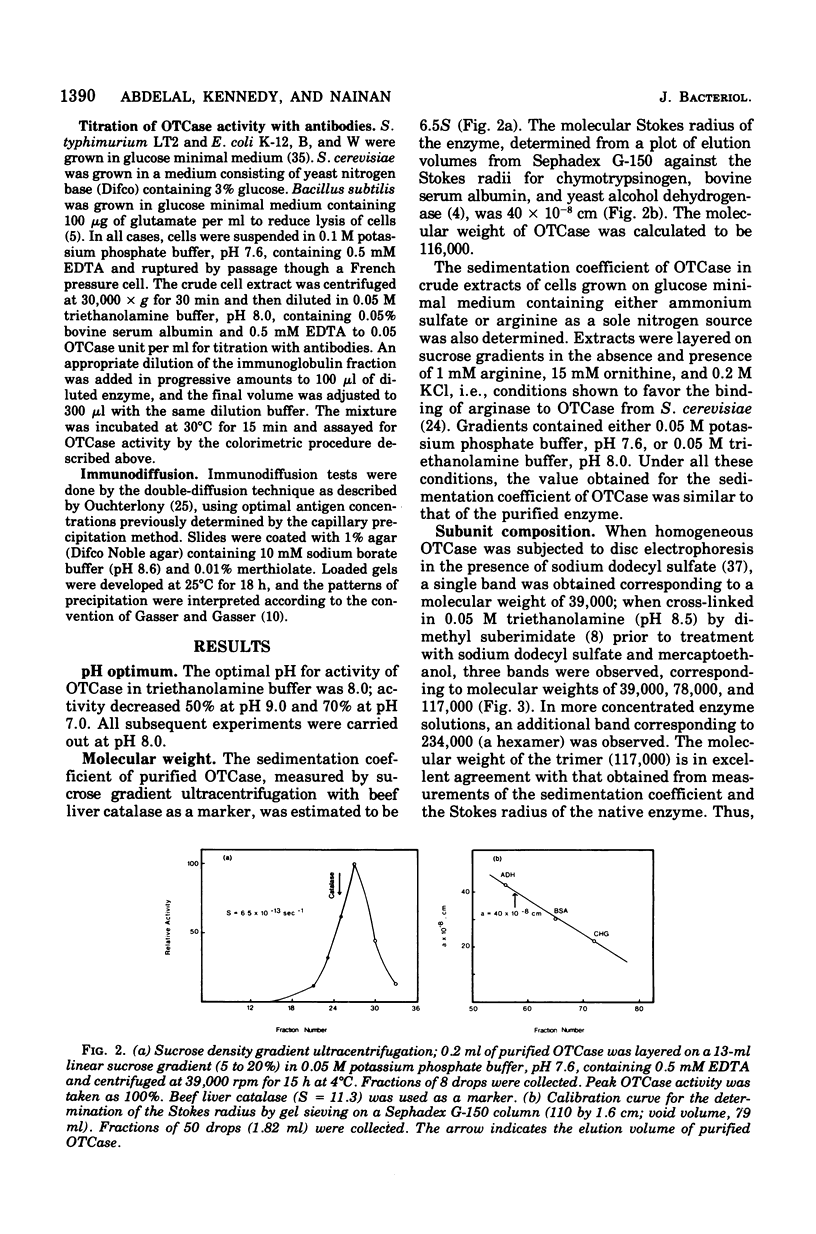
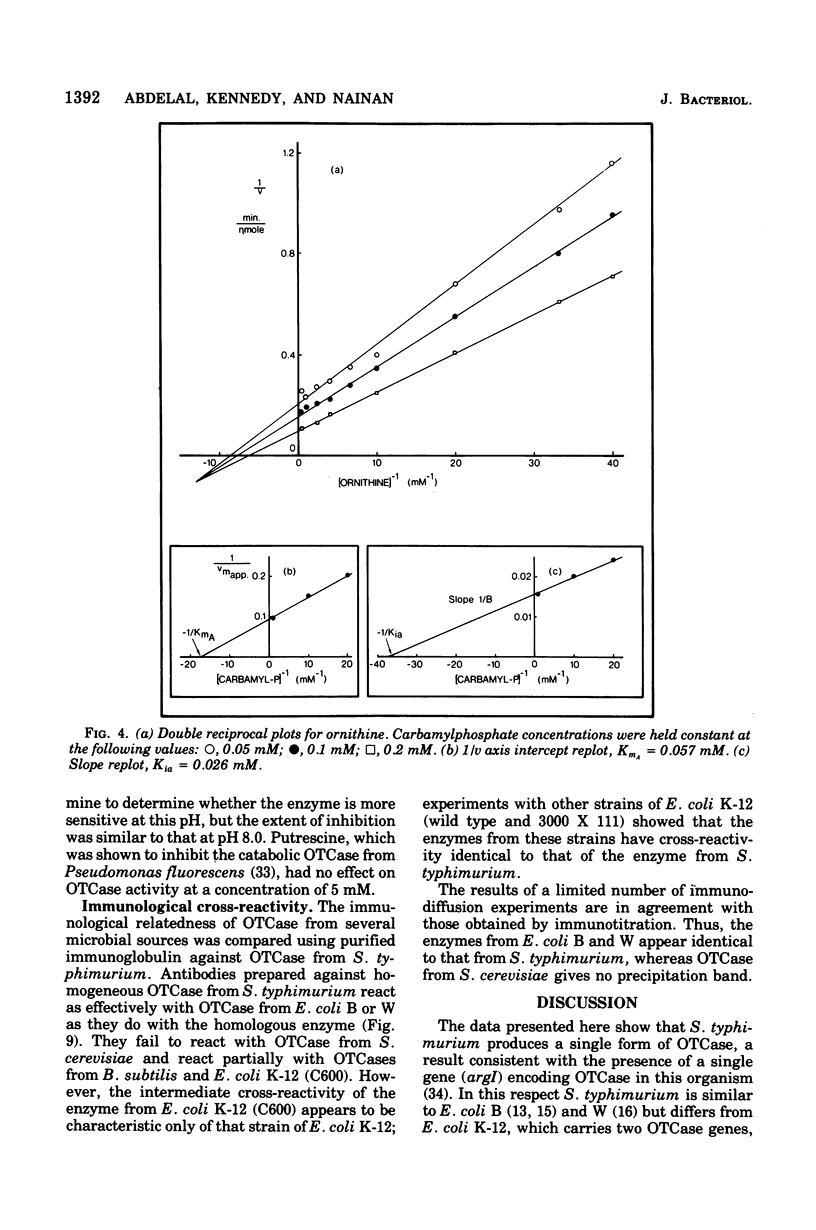
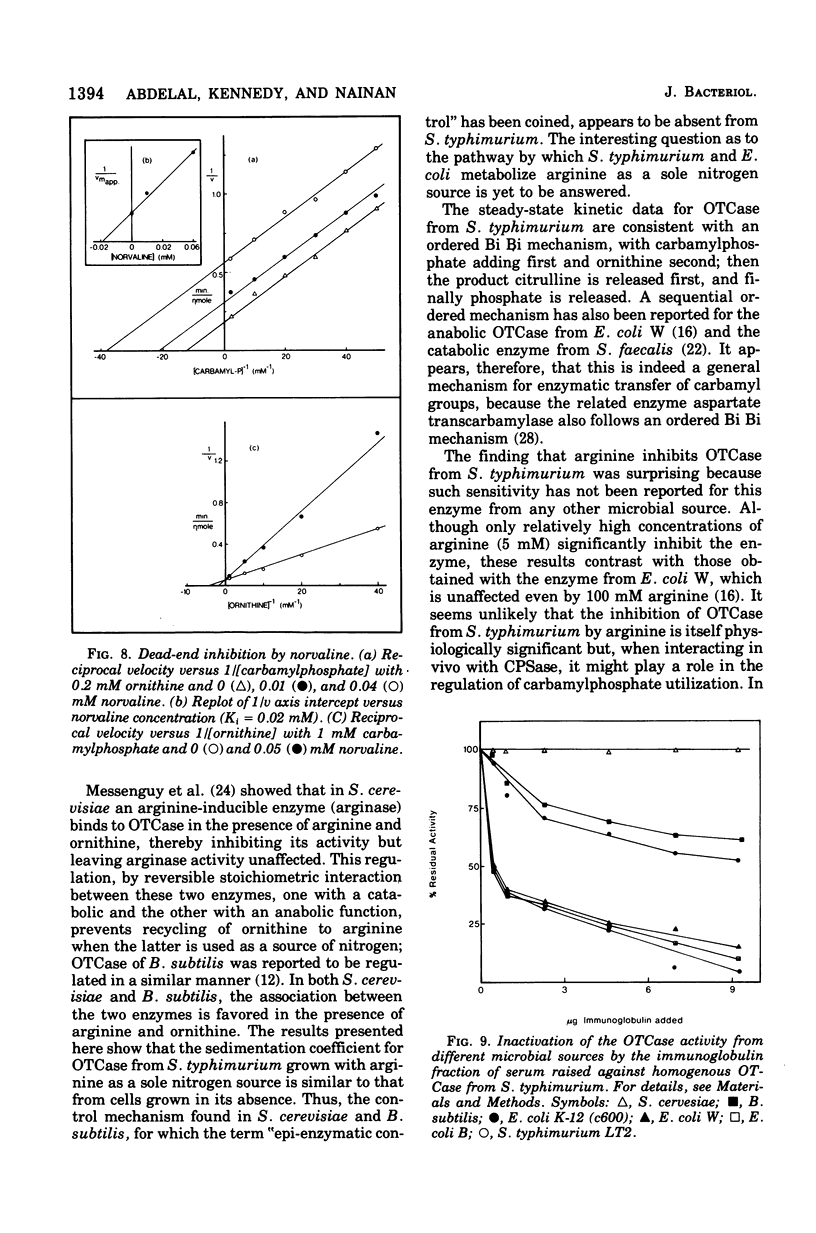
Ornithine transcarbamylase (OTCase) was purified to hemogeneity from a derepressed strain of Salmonella typhimurium. The optimal pH for enzyme activity is 8.0. The molecular weight of the enzyme was calculated to be 116,000, based on measurements of the sedimentation coefficient by sucrose gradient ultracentrifugation and the Stokes radius by gel filtration. Polyacrylamide gel electrophoresis of cross-linked OTCase in the presence of sodium dodecyl sulfate showed that the enzyme is composed of three identical subunits. The molecular weight of the monomer was determined to be 39,000. Steady-state kinetics indicate that the reaction mechanism is sequential. The limiting Michealis constants for carbamylphosphate and ornithine were determined to be 0.06 and 0.2 mM, respectively. The dissociation constant for carbamylphosphate was 0.02 mM. Product and dead-end inhibition patterns are consistent with an ordered Bi Bi mechanism, in which carbamylphosphate is the first substrate added and phosphate is the last product released. OTCase activity was inhibited by arginine, but relatively high concentrations were required for significant inhibition. The inhibition by arginine might be physiologically significant in the regulation of carbamlphosphate utilization; a single carbamylphosphate synthetase is responsible for the synthesis of carbamylphosphate for both arginine and pyrimidines in S. typhimurium and the inhibition by argine might serve to divert carbamlphosphate to the synthesis of pyrimidines when arginine is present at high concentrations. The crossreaction of OTCases from different microorganisms with purified antibodies raised against the homogeneous OTCase from S. typhimurium was investigated. The results of immunotitration and immunodiffusion experiments revealed a high degree of identity between the enzymes form S. typhimurium and Esherichia coli B and W. In these three cases, a single gen (argl) encodes OTCase. Wild-type E. coli K-12 and strain 3000 X 111, which carry two OTCase genes (argI, argF), also revealed similar cross-reactivity, supporting the hypothesis that argF is the product of a relatively recent duplication. The activity of OTCase from Bacillus subtilis was partially inhibited by antibodies against the enzyme from S. typhimurium, indicating unusual conservation of primary structure among widely different taxonomic groups. OTCase from Saccharomyces cerevisiae, whose molecular weight and primary structure are similar to those of the enzyme from S. typhimurium, was without detectable cross-reactivity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Abdelal A. T., Griego E., Ingraham J. L. Arginine-sensitive phenotype of mutations in pyrA of Salmonella typhimurium: role of ornithine carbamyltransferase in the assembly of mutant carbamylphosphate synthetase. J Bacteriol. 1976 Oct;128(1):105–113. doi: 10.1128/jb.128.1.105-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Kelker N., Boyen A., Yang H., Zubay G., Glansdorff N., Maas W. K. Involvement of arginine in in vitro repression of transcription of arginine genes C, B and H in Escherichia coli K 12. Biochem Biophys Res Commun. 1976 Mar 22;69(2):377–382. doi: 10.1016/0006-291x(76)90532-5. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser F., Gasser C. Immunological relationships among lactic dehydrogenases in the genera Lactobacillus and Leuconostoc. J Bacteriol. 1971 Apr;106(1):113–125. doi: 10.1128/jb.106.1.113-125.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleux P., Legrain C., Stalon V., Piérard A., Wiame J. M. Regulation of the catabolic ornithine carbamoyltransferase of Pseudomonas fluorescens. A study of the quaternary structure. Eur J Biochem. 1972 Dec 4;31(2):386–393. doi: 10.1111/j.1432-1033.1972.tb02545.x. [DOI] [PubMed] [Google Scholar]
- Issaly I. M., Issaly A. S. Control of ornithine carbamoyltransferase activityby arginase in Bacillus subtilis. Eur J Biochem. 1974 Dec 2;49(3):485–495. doi: 10.1111/j.1432-1033.1974.tb03853.x. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Mapping the gene determining ornithine transcarbamylase and its operator in Escherichia coli B. J Bacteriol. 1971 Nov;108(2):645–651. doi: 10.1128/jb.108.2.645-651.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelln R. A., Foltermann K. F., O'Donovan G. A. Location of the argR gene on the chromosome of Salmonella typhimurium. Mol Gen Genet. 1975 Sep 8;139(4):277–284. doi: 10.1007/BF00267967. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legrain C., Halleux P., Stalon V., Glansdorff N. The dual genetic control of ornithine carbamolytransferase in Escherichia coli. A case of bacterial hybrid enzymes. Eur J Biochem. 1972 May;27(1):93–102. doi: 10.1111/j.1432-1033.1972.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Legrain C., Stalon V., Glansdorff N. Escherichia coli ornithine carbamolytransferase isoenzymes: evolutionary significance and the isolation of lambdaargF and lambdaargI transducing bacteriophages. J Bacteriol. 1976 Oct;128(1):35–38. doi: 10.1128/jb.128.1.35-38.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain C., Stalon V. Ornithine carbamoyltransferase from Escherichia coli W. Purification, structure and steady-state kinetic analysis. Eur J Biochem. 1976 Mar 16;63(1):289–301. doi: 10.1111/j.1432-1033.1976.tb10230.x. [DOI] [PubMed] [Google Scholar]
- London J., Kline K. Aldolase of lactic acid bacteria: a case history in the use of an enzyme as an evolutionary marker. Bacteriol Rev. 1973 Dec;37(4):453–478. doi: 10.1128/br.37.4.453-478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. II. Multiple binding sites for carbamyl-P and L-norvaline, correlation with steady state kinetics. J Biol Chem. 1972 Mar 25;247(6):1654–1668. [PubMed] [Google Scholar]
- Messenguy F., Penninckx M., Wiame J. M. Interaction between arginase and ornithine carbamoyltransferase in Saccharomyces cerevisiae. The regulatory site for ornithine. Eur J Biochem. 1971 Sep 24;22(2):277–286. doi: 10.1111/j.1432-1033.1971.tb01542.x. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Penninckx M., Simon J. P., Wiame J. M. Interaction between arginase and L-ornithine carbamoyltransferase in Saccharomyces cerevisiae. Purification of S. cerevisiae enzymes and evidence that these enzymes as well as rat-liver arginase are trimers. Eur J Biochem. 1974 Nov 15;49(2):429–442. doi: 10.1111/j.1432-1033.1974.tb03848.x. [DOI] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. II. Comparison of precipitin and micro-complement fixation results. J Biol Chem. 1971 Nov 25;246(22):7010–7017. [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Berlin C. M., Sweeney E. W., Carroll W. R. The generation of energy by the arginine dihydrolase pathway in Mycoplasma hominis 07. J Biol Chem. 1966 May 25;241(10):2228–2236. [PubMed] [Google Scholar]
- Stalon V., Ramos F., Piérard A., Wiame J. M. Regulation of the catabolic ornithine carbamoyltransferase of Pseudomonas fluorescens. A comparison with the anabolic transferase and with a mutationally modified catabolic transferase. Eur J Biochem. 1972 Aug 18;29(1):25–35. doi: 10.1111/j.1432-1033.1972.tb01953.x. [DOI] [PubMed] [Google Scholar]
- Syvanen J. M., Roth J. R. Structural genes for ornithine transcarbamylase in Salmonella typhimurium and Escherichia coli K-12. J Bacteriol. 1972 Apr;110(1):66–70. doi: 10.1128/jb.110.1.66-70.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



