Abstract
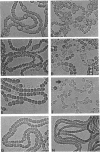
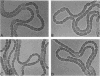
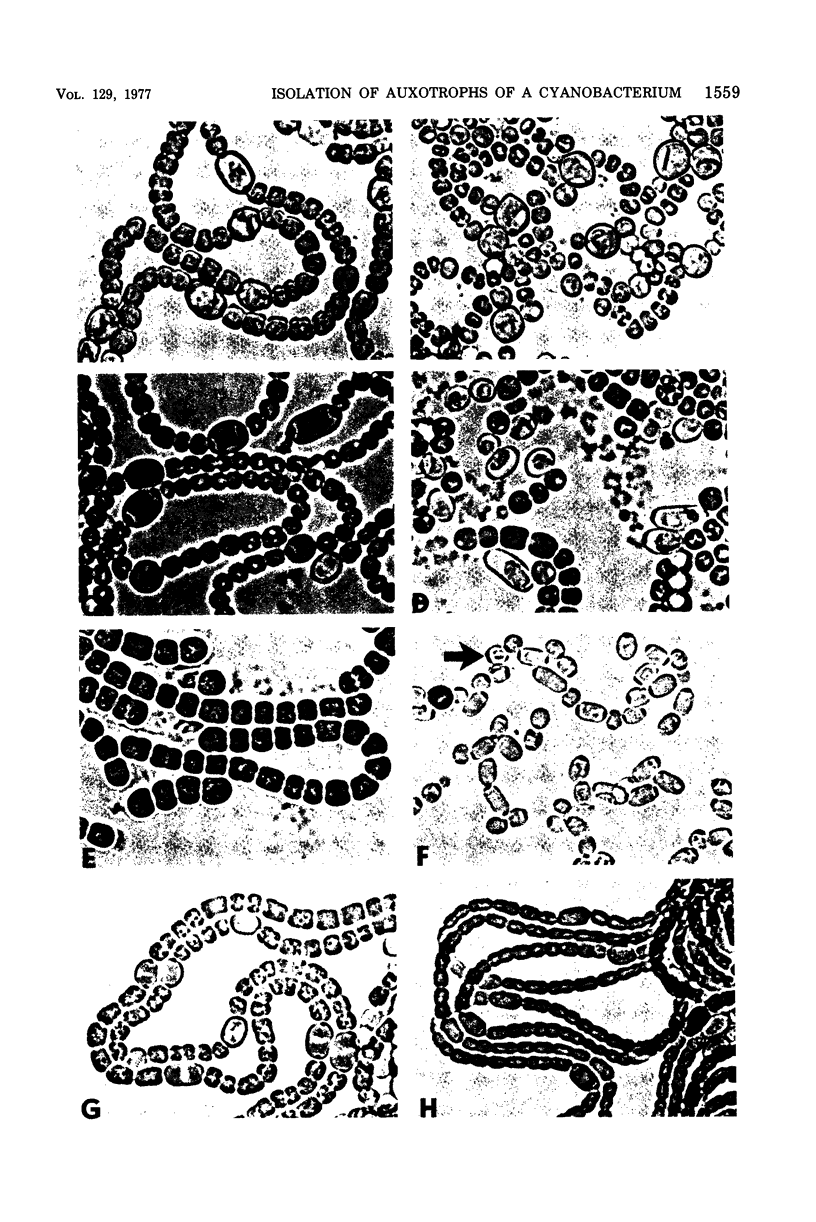
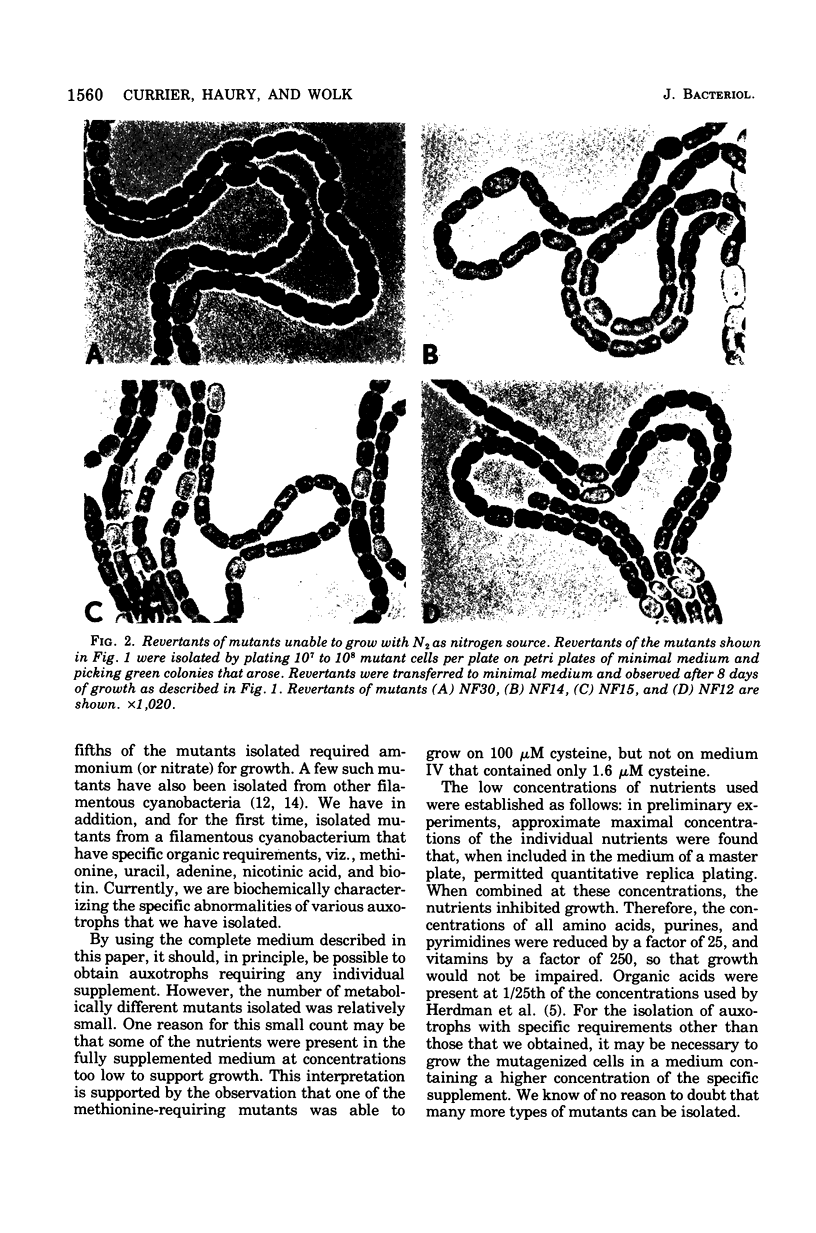
Auxotrophic mutants of the filamentous cyanobacterium Anabaena variabilis were isolated by a method in which, after mutagenesis and before penicllin enrichment, mutant and wild-type cells were separated by cavitation. Auxotrophs were identified by their inability to grow on minimal medium, and they were partially characterized by replica plating to media supplemented with single nutrients or specific groups of nutrients. Of the 83 auxotrophs isolated, 65 required an inorganic source of nitrogen for growth. In addition, auxotrophs were isolated that required methionine (six), uracil (two), adenine (one), biotin (two), and nicotinic acid (two). (The number of isolates of each type is indicated in parentheses.) The nutrient requirements of five auxotrophs appeared complex and were not determined. A large proportion of the mutants requiring inorgainic fixed nitrogen was altered in the differentiation of heterocysts. The following morphological aberrancies were observed: abnormally high and abnormally low frequencies of heterocysts; thick, uneven heterocyst envelopes; incompletely developed pore regions; very distinct pore regions; and protoplasts separated from the envelope of the heterocyst. Spontaneously occurring, N2-fixing, prototrophic revertants of mutants with aberrant heterocysts have been isolated at a frequency of 2 X 10(-8) to 4 X 10(-8) of the cells plated. That most such revertants produced morphologically normal heterocysts is consisten with the idea that heterocysts play an essential role in aerobic N2 fixation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN A. C., WOOD H. N. On the activation of certain essential biosynthetic systems in cells of Vinca rosea L. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1776–1782. doi: 10.1073/pnas.48.10.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman M., Carr N. G. The isolation and characterization of mutant strains of the blue-green alga anacystis nidulans. J Gen Microbiol. 1972 Apr;70(2):213–220. doi: 10.1099/00221287-70-2-213. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Pierson D., Kane J. F., Van Baalen C., Jensen R. A. Documentation of auxotrophic mutation in blue-green bacteria: characterization of a tryptophan auxotroph in Agmenellum quadruplicatum. J Bacteriol. 1972 Jul;111(1):112–118. doi: 10.1128/jb.111.1.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Van Baalen C. Characteristics of a stable, filamentous mutant of a coccoid blue-green alga. J Bacteriol. 1970 Jun;102(3):784–789. doi: 10.1128/jb.102.3.784-789.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Shilo M. Short-trichome mutant of Plectonema boryanum. J Bacteriol. 1969 Feb;97(2):975–976. doi: 10.1128/jb.97.2.975-976.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Burris R. H. Properties of heterocysts isolated with colloidal silica. Arch Microbiol. 1976 May 3;108(1):35–40. doi: 10.1007/BF00425090. [DOI] [PubMed] [Google Scholar]
- Stevens C. L., Stevens S. E., Jr, Myers J. Isolation and initial characterization of a uracil auxotroph of the blue-green alga Anacystis nidulans. J Bacteriol. 1975 Oct;124(1):247–251. doi: 10.1128/jb.124.1.247-251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D., Singh H. N. Transfer of nitrogen-fixing (NIF) genes in the blue-green alga Nostoc muscorum. Biochem Biophys Res Commun. 1975 Jan 6;62(1):62–69. doi: 10.1016/s0006-291x(75)80405-0. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Stewart W. D. Photosynthetic electron transport, ATP synthesis and nitrogenase activity in isolated heterocysts of Anabaena cylindrica. Biochim Biophys Acta. 1976 Feb 16;423(2):189–195. doi: 10.1016/0005-2728(76)90177-8. [DOI] [PubMed] [Google Scholar]
- Thomas J., Meeks J. C., Wolk C. P., Shaffer P. W., Austin S. M. Formation of glutamine from [13n]ammonia, [13n]dinitrogen, and [14C]glutamate by heterocysts isolated from Anabaena cylindrica. J Bacteriol. 1977 Mar;129(3):1545–1555. doi: 10.1128/jb.129.3.1545-1555.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox M., Mitchison G. J., Smith R. J. Mutants of Anabaena cylindrica altered in heterocyst spacing. Arch Microbiol. 1975 May 5;103(3):219–223. doi: 10.1007/BF00436353. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Austin S. M., Bortins J., Galonsky A. Autoradiographic localization of 13N after fixation of 13N-labeled nitrogen gas by a heterocyst-forming blue-green alga. J Cell Biol. 1974 May;61(2):440–453. doi: 10.1083/jcb.61.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P., Wojciuch E. Simple methods for plating single vegetative cells of, and for replica-plating, filamentous blue-green algae. Arch Mikrobiol. 1973 May 24;91(2):91–95. doi: 10.1007/BF00424753. [DOI] [PubMed] [Google Scholar]