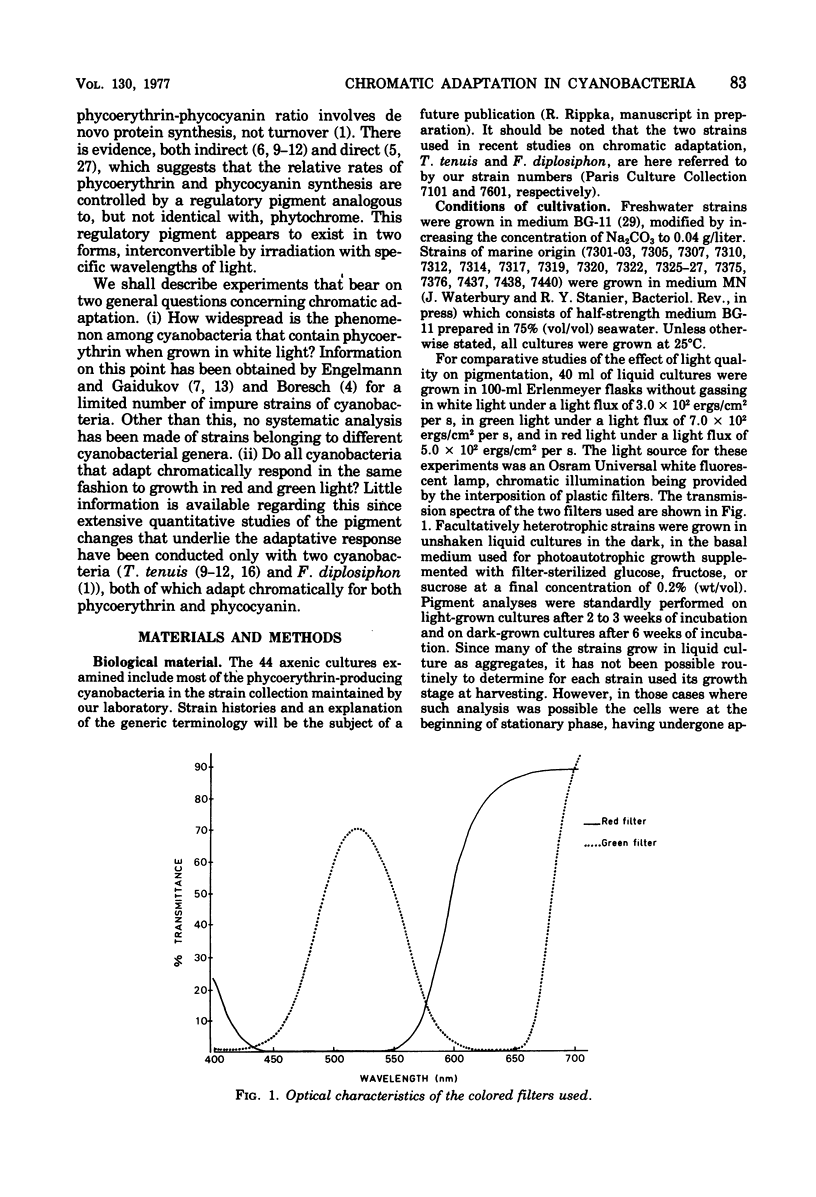
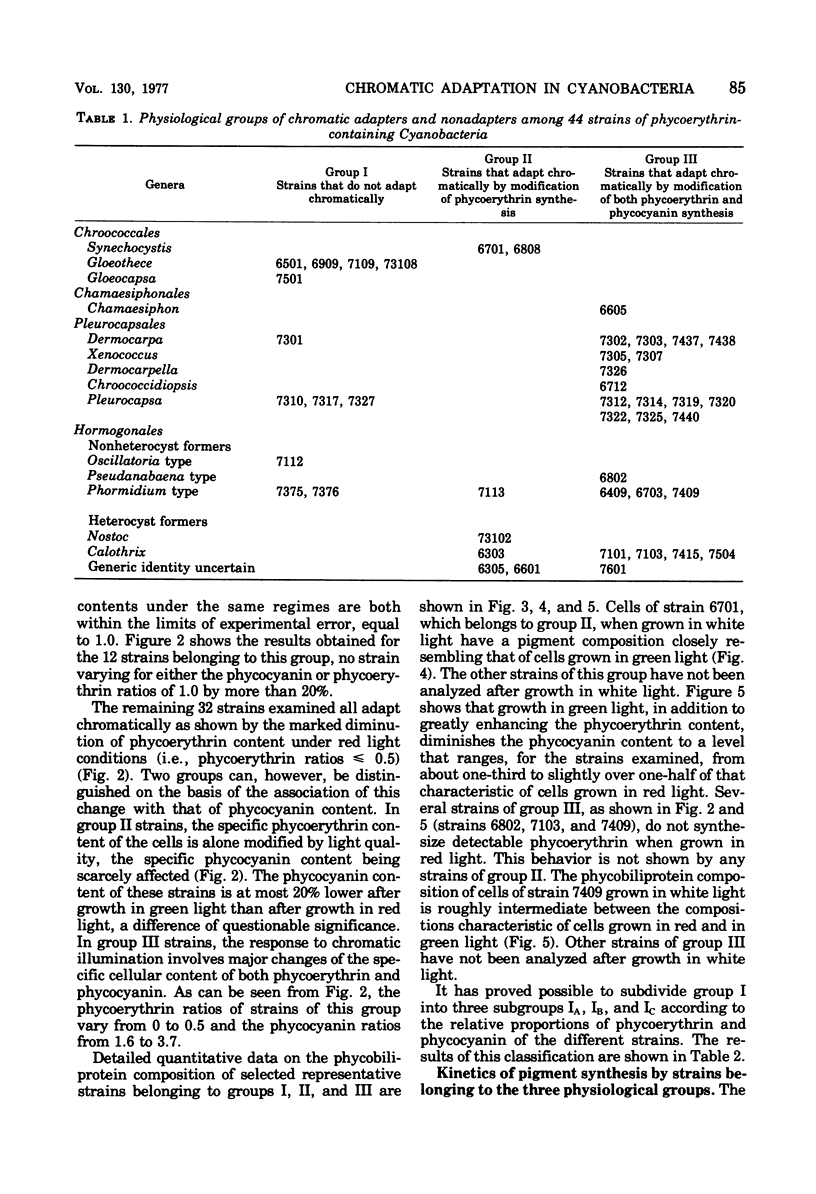
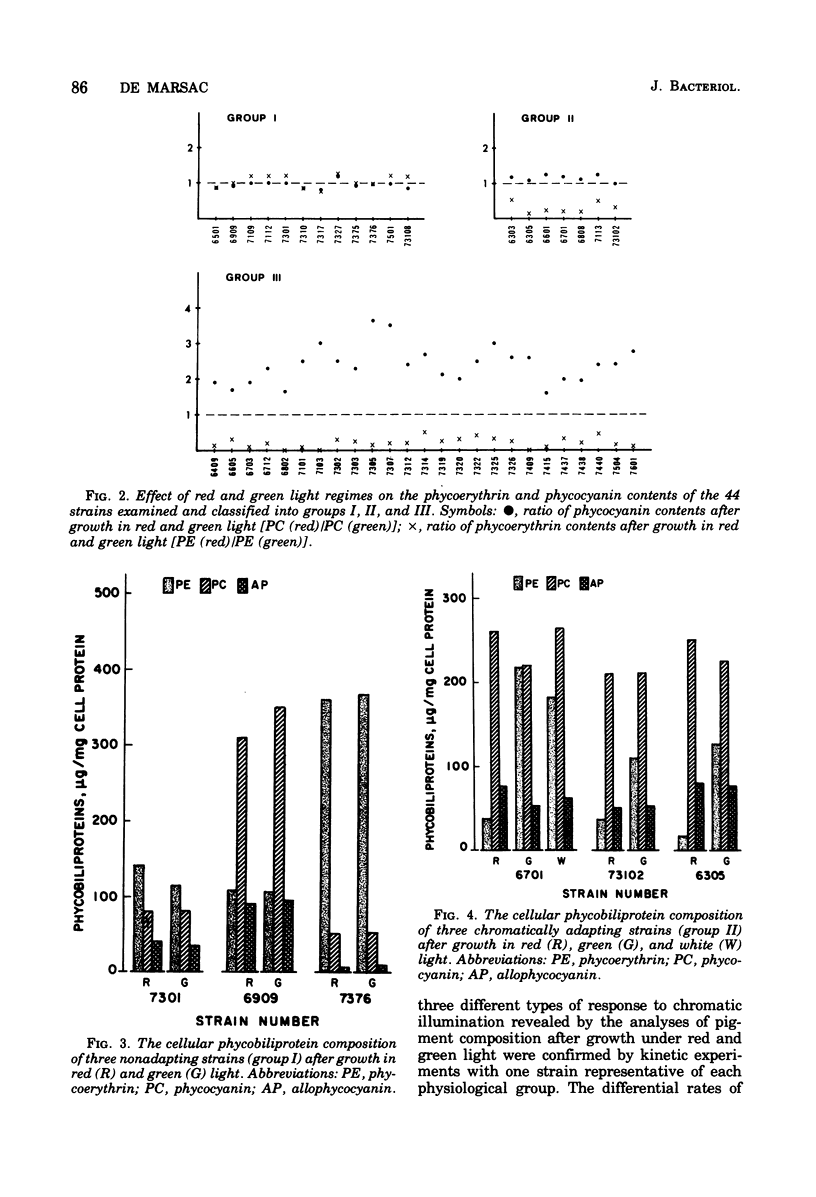
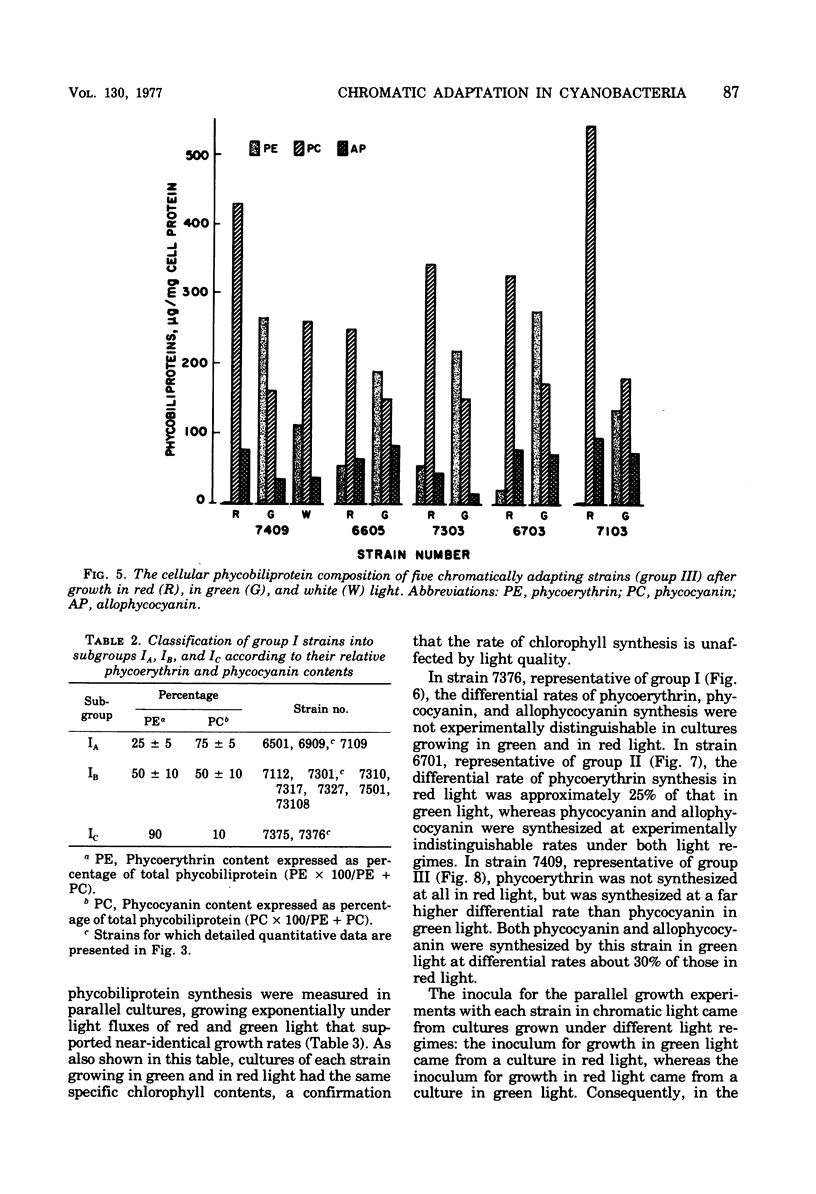
Abstract
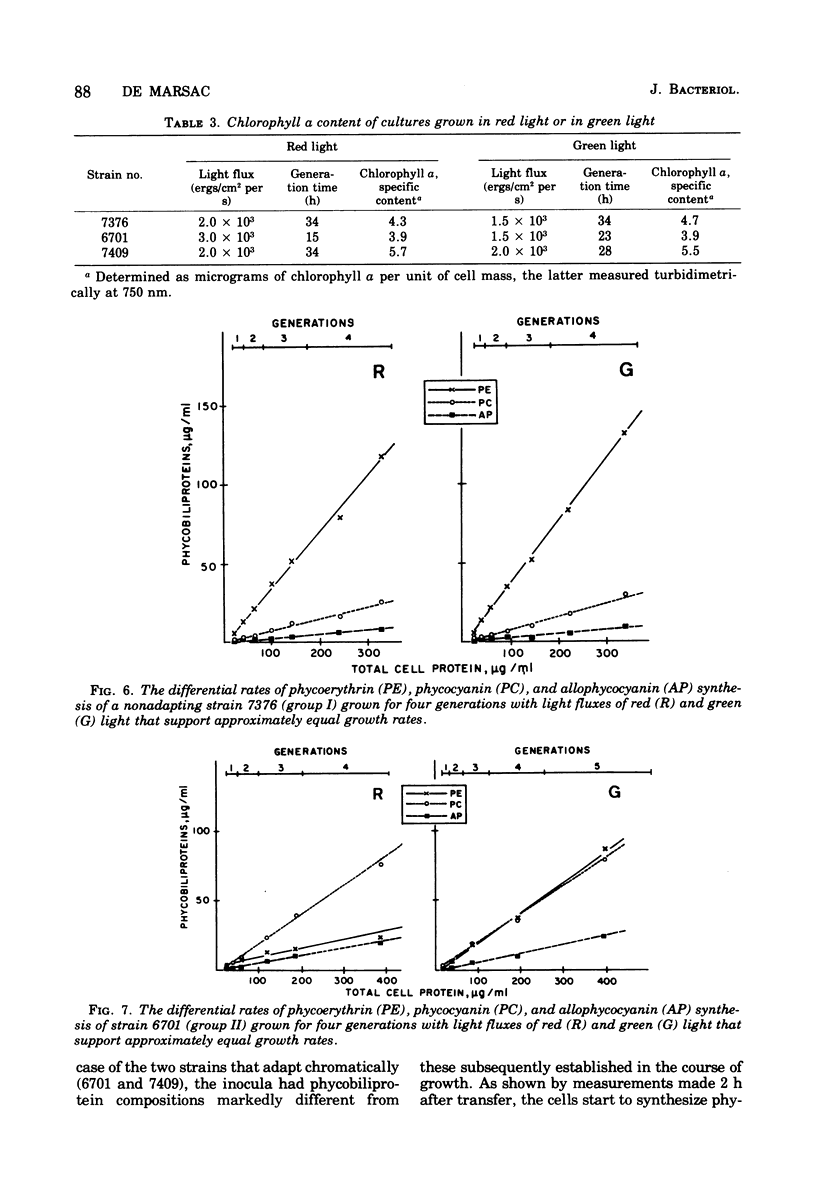
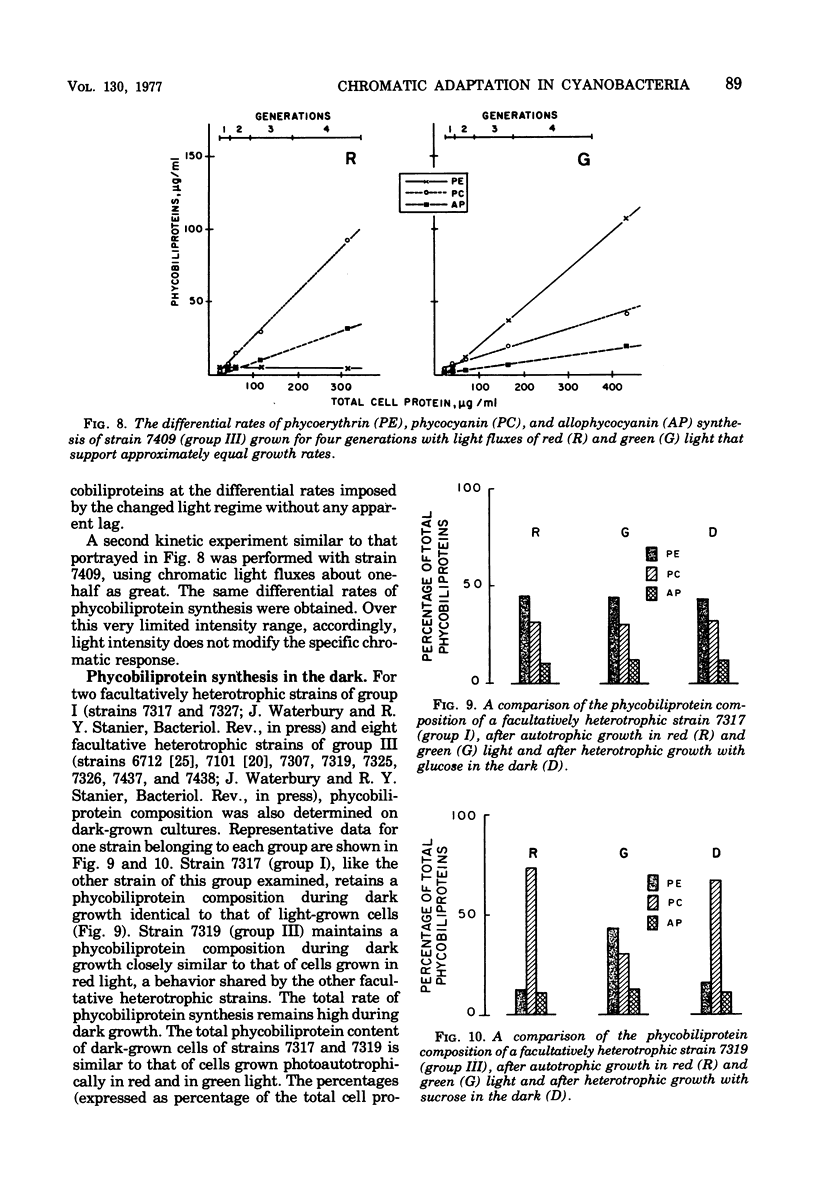
Forty-four axenic strains of cyanobacteria that synthesize phycoerythrin were screened to ascertain the effect of light quality on pigment synthesis. Cellular pigment compositions were determined after photoautotrophic growth with low light fluxes (7.0 X 10(2) ergs/cm2 per s) of green, red, and white light, and in the case of facultative heterotrophs, after dark growth at the expense of sugars. Twelve strains did not adapt chromatically: the cells contained fixed proportions of phycoerythrin, phycocyanin, and allophycocyanin under the growth conditions used. In the remaining strains, the cellular ratio of phycoerythrin to phycocyanin was much higher after growth in green than in red light. Quantitative data on the cellular pigment contents, supplemented by measurements of the differential rates of pigment synthesis on representative strains, show that chromatic adaptation may involve a light-induced modulation either of phycoerythrin synthesis alone (7 strains) or of both phycoerythrin and phycocyanin synthesis (25 strains). Facultative hetrotrophs able to adapt chromatically have a phycobiliprotein composition after dark growth which closely resembles that after growth in red light. Light quality does not affect the differential rate of chlorophyll synthesis. The physiological and taxonomic implications of these findings are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A., Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973 Aug;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakoff S., Scheibe J. Action Spectra for Chromatic Adaptation in Tolypothrix tenuis. Plant Physiol. 1973 Feb;51(2):382–385. doi: 10.1104/pp.51.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Hixson C. S. Characterization of R-phycocyanin. Chromophore content of R-phycocyanin and C-phycoerythrin. J Biol Chem. 1975 Jul 25;250(14):5487–5495. [PubMed] [Google Scholar]
- Jones L. W., Myers J. Enhancement in the Blue-Green Alga, Anacystis nidulans. Plant Physiol. 1964 Nov;39(6):938–946. doi: 10.1104/pp.39.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemasson C., Marsac N. T., Cohen-Bazire G. Role of allophycocyanin as light-harvesting pigment in cyanobacteria. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3130–3133. doi: 10.1073/pnas.70.11.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., PAPPENHEIMER A. M., Jr, COHEN-BAZIRE G. La cinétique de la biosynthèse de la beta-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim Biophys Acta. 1952 Dec;9(6):648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- Scheibe J. Photoreversible pigment: occurrence in a blue-green alga. Science. 1972 Jun 2;176(4038):1037–1039. doi: 10.1126/science.176.4038.1037. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


