Abstract
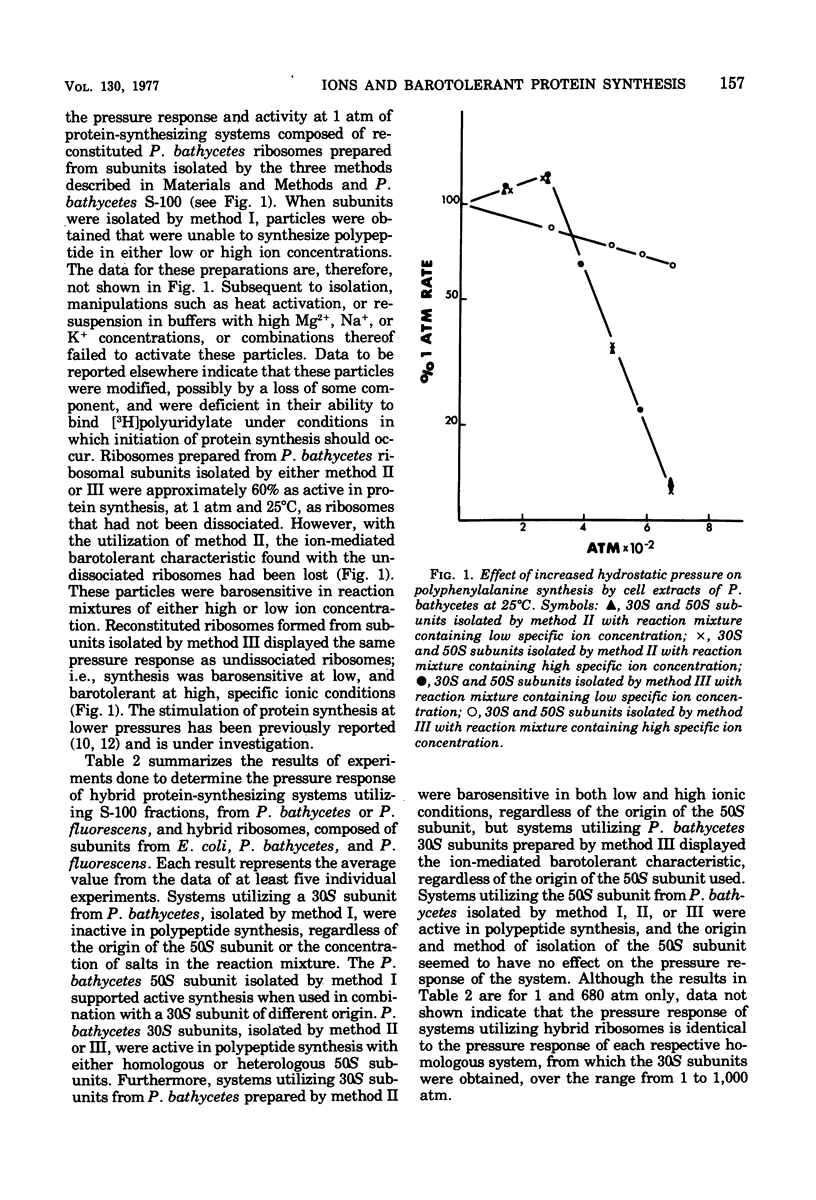
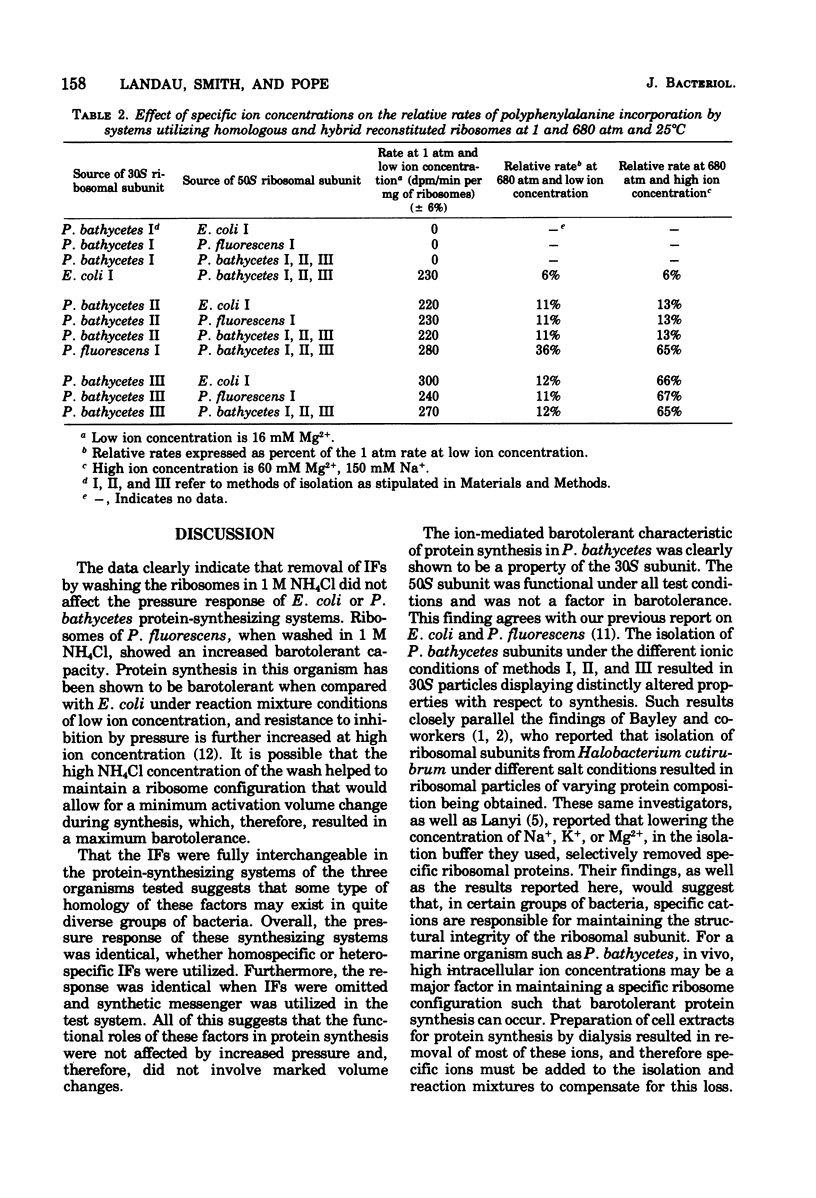
Washed (1 M NH4Cl) ribosomes from Pseudomonas bathycetes, Pseudomonas fluorescens, and Escherichia coli were tested for their ability to synthesize protein or polypeptide at high pressure when used as such, when recombined with homologous initiation factors, and when recombined with heterologous initiation factors. The responses of natural messenger ribonucleic acid (MS-2)-directed systems to pressure were independent of the source of initiation factors and paralleled those of the washed ribosomes in polyuridylate-directed systems. In all cases, the responses to pressure were parallel to those obtained when unwashed ribosomes were utilized; therefore, we concluded that the initiation factors were interchangeable among these organisms, and that these factors did not play a critical role in determining the pressure responses of the protein-synthesizing systems. P. bathycetes ribosomal subunits were isolated under a variety of ionic conditions. These were tested for their ability to synthesize protein and polyphenylalanine at a variety of pressures when used in reconstituted P. bathycetes homologous systems and in hybrid systems with ribosomal subunits from E. coli and P. fluorescens. O. bathycetes 30S subunits, isolated in a buffer solution containing 0 mM NaCl and O mM KC] were functional at any pressure; those isolated in the presence of 150 mM NaCl and 0 mM KCl were functional at 1 atmosphere but barosensitive, and those isolated in the presence of O mM NaCl and 150 mM KCl retained the ion-mediated barotolerance characteristic of crude P. bathycetes ribosome preparations. The 50S subunit remained functional regardless of the method of isolation, and it had no effect on pressure sensitivity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAYLEY S. T., KUSHNER D. J. THE RIBOSOMES OF THE EXTREMELY HALOPHILIC BACTERIUM, HALOBACTERIUM CUTIRUBRUM. J Mol Biol. 1964 Sep;9:654–669. doi: 10.1016/s0022-2836(64)80173-x. [DOI] [PubMed] [Google Scholar]
- Bayley S. T. Composition of ribosomes of an extremely halophilic bacterium. J Mol Biol. 1966 Feb;15(2):420–427. doi: 10.1016/s0022-2836(66)80117-1. [DOI] [PubMed] [Google Scholar]
- Gottlieb M., Davis B. D. The irreversible step in formation of initiation complexes of Escherichia coli. Biochemistry. 1975 Mar 11;14(5):1047–1051. doi: 10.1021/bi00676a025. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Baierlein R. Pressure-induced dissociation of sedimenting ribosomes: effect on sedimentation patterns. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1780–1785. doi: 10.1073/pnas.68.8.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanyi J. K. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol Rev. 1974 Sep;38(3):272–290. doi: 10.1128/br.38.3.272-290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. S., Somero G. N. Activation volumes in enzymic catalysis: their sources and modification by low-molecular-weight solutes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3014–3018. doi: 10.1073/pnas.72.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. S., Somero G. N. Protein hydration changes during catalysis: a new mechanism of enzymic rate-enhancement and ion activation/inhibition of catalysis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3305–3309. doi: 10.1073/pnas.72.9.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope D. H., Smith W. P., Swartz R. W., Landau J. V. Role of bacterial ribosomes in barotolerance. J Bacteriol. 1975 Feb;121(2):664–669. doi: 10.1128/jb.121.2.664-669.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Landau J. V. Hydrostatic pressure effects on Escherichia coli: site of inhibition of protein synthesis. J Bacteriol. 1972 Feb;109(2):945–948. doi: 10.1128/jb.109.2.945-948.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Landau J. V. Inhibition of cell-free protein synthesis by hydrostatic pressure. J Bacteriol. 1972 Dec;112(3):1222–1227. doi: 10.1128/jb.112.3.1222-1227.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Landau J. V., Pope D. H. Specific ion concentration as a factor in barotolerant protein synthesis in bacteria. J Bacteriol. 1976 May;126(2):654–660. doi: 10.1128/jb.126.2.654-660.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W., Pope D., Landau J. V. Role of bacterial ribosome subunits in barotolerance. J Bacteriol. 1975 Oct;124(1):582–584. doi: 10.1128/jb.124.1.582-584.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


