Abstract
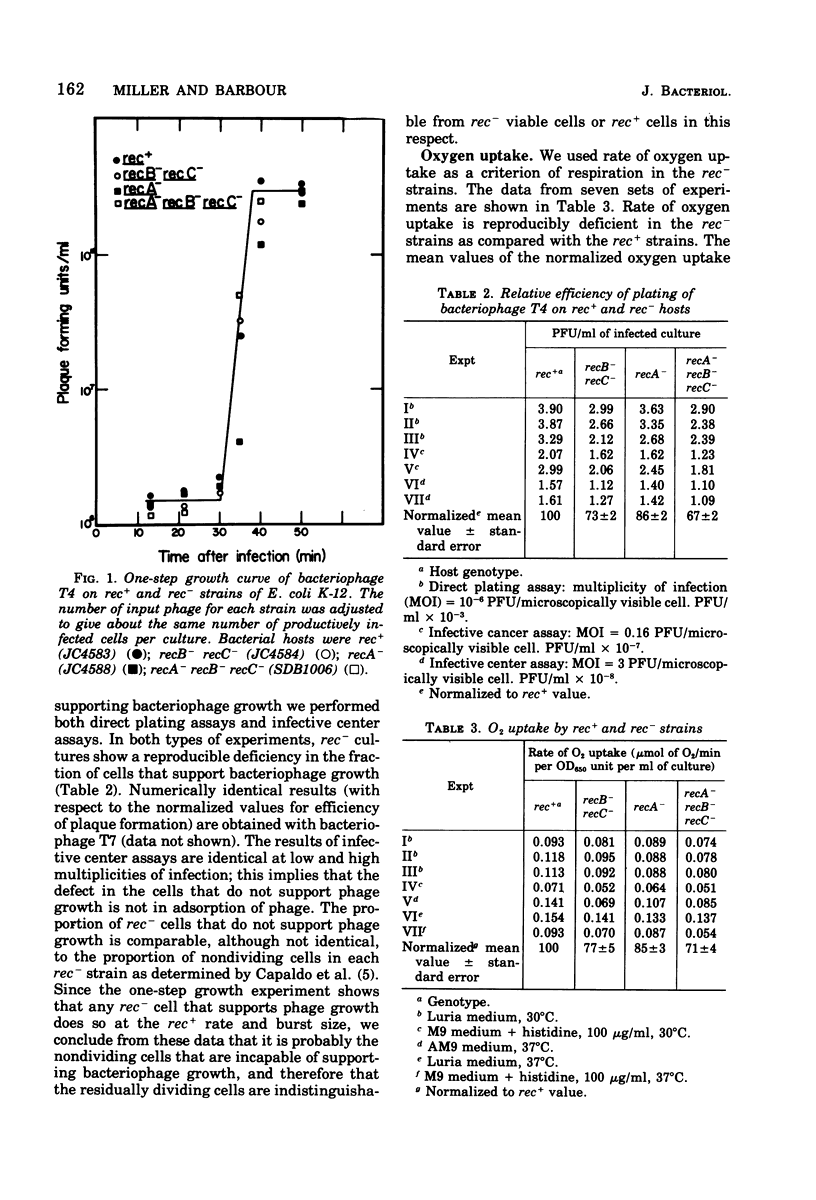
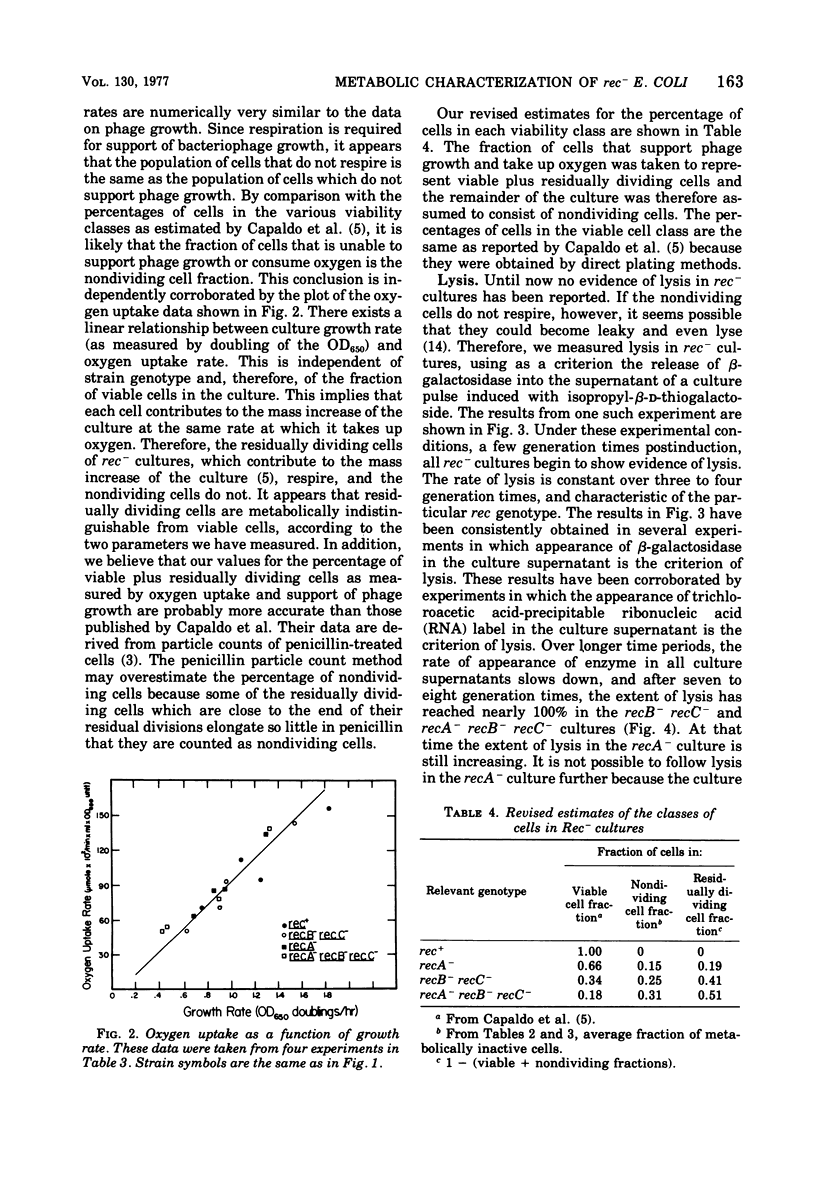
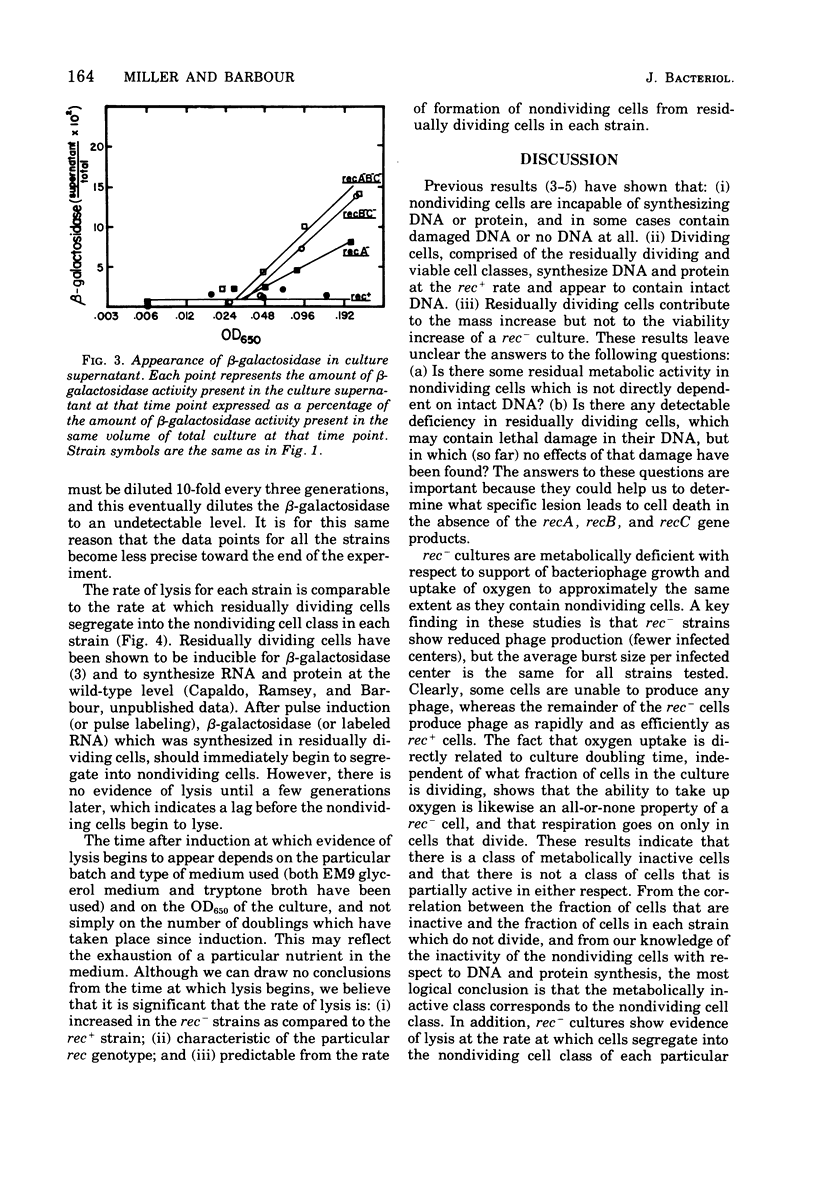
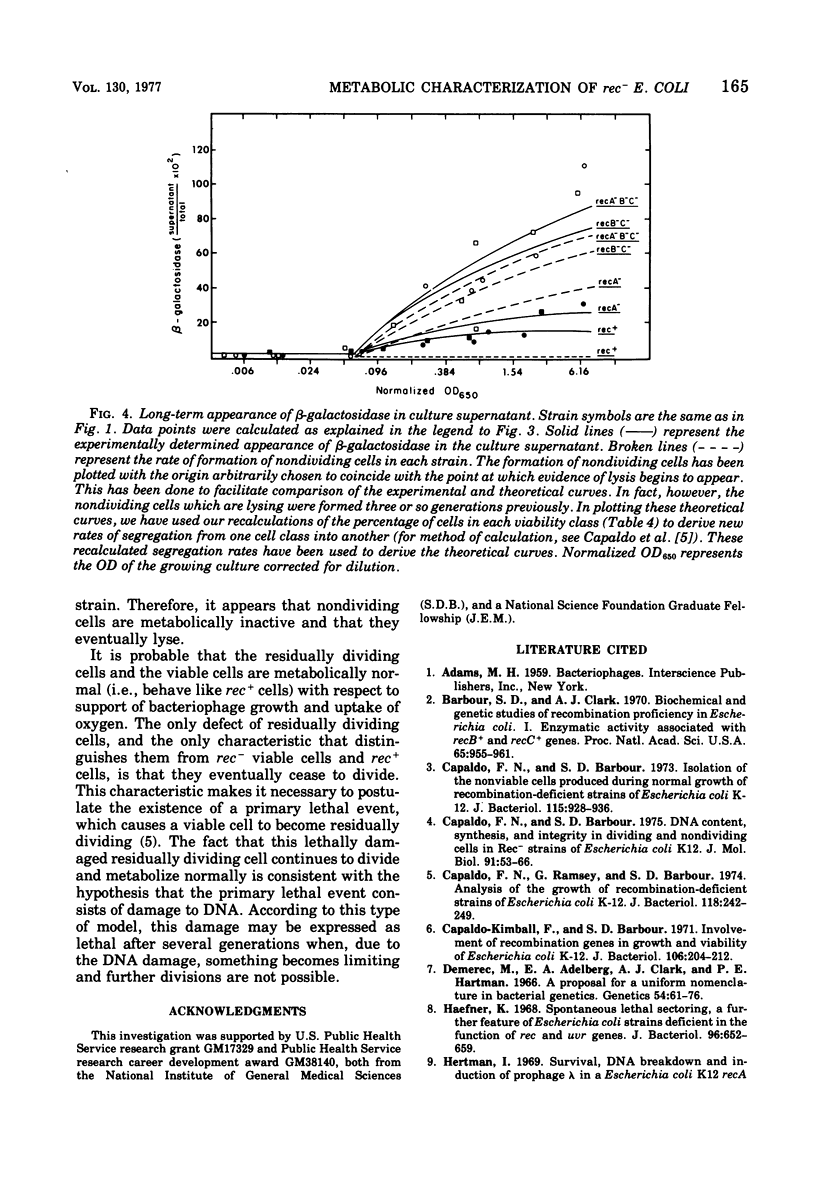
The abilities of rec+, recB- recC-, recA-, and recA- recB- rec C- strains to support growth of bacteriophage T4, to take up oxygen, and to maintain cell integrity have been measured. (i) With respect to bacteriophage T4 growth, T4 phage is produced with identical lysis time in single -step growth curves with all strains tested. rec- strains show reduced phage production (fewer infected centers), but the average burst size per infected center is the same for all strains tested. Some rec- cells are unable to produce any phage, whereas the remainder of the rec-cells produce phage as rapidly and as efficiently as rec+ cells. (ii) With respect to oxygen consumption, rec- strains are deficient relative to the rec+ control to the same extent as the deficiency in phage production by theculture. The reduction in oxygen consumption is coordinate with reduction in rate of mass increase of the rec- culture. (iii) With respect to cell integrity, rec- cultures show increased lysis as measured by release of beta-galactosidase into the medium. The kinetics of release suggest that rec- nondividing cells all eventually lyse. These results are most consistent with the idea that rec- residually dividing cells and viable cells are metabolically normal according to the parameters we have measured, whereas nondividing cells are metabolically inactive.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo F. N., Barbour S. D. DNA content, synthesis and integrity in dividing and non-dividing cells of rec- strains of Escherichia coli K12. J Mol Biol. 1975 Jan 5;91(1):53–66. doi: 10.1016/0022-2836(75)90371-x. [DOI] [PubMed] [Google Scholar]
- Capaldo F. N., Barbour S. D. Isolation of the nonviable cells produced during normal growth of recombination-deficient strains of Escherichia coli K-12. J Bacteriol. 1973 Sep;115(3):928–936. doi: 10.1128/jb.115.3.928-936.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo F. N., Ramsey G., Barbour S. D. Analysis of the growth of recombination-deficient strains of Escherichia coli K-12. J Bacteriol. 1974 Apr;118(1):242–249. doi: 10.1128/jb.118.1.242-249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefner K. Spontaneous lethal sectoring, a further feature of Escherichia coli strains deficient in the function of rec and uvr genes. J Bacteriol. 1968 Sep;96(3):652–659. doi: 10.1128/jb.96.3.652-659.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Pleiotropic effect of the rec A gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J Bacteriol. 1971 May;106(2):539–542. doi: 10.1128/jb.106.2.539-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Evidence relating cessation of respiration, cell envelope changes, and death in ultraviolet-irradiated Escherichia coli B-r cells. J Bacteriol. 1974 Feb;117(2):551–559. doi: 10.1128/jb.117.2.551-559.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Recombination and the Escherichia coli K-12 sex factor F. J Bacteriol. 1975 Jan;121(1):36–43. doi: 10.1128/jb.121.1.36-43.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


